Drug Combination Details
| General Information of the Combination (ID: C69078) | |||||
|---|---|---|---|---|---|
| Name | Voglibose NP Info | + | Alogliptin Drug Info | ||
| Structure |
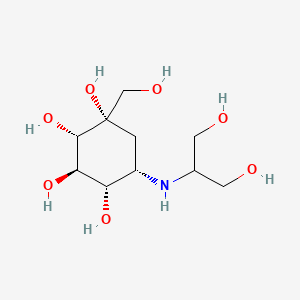
|
+ |
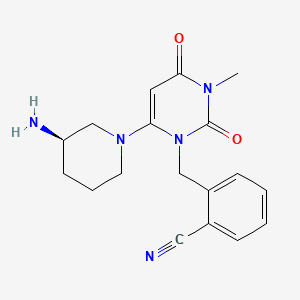
|
||
| Disease |
Type 2 diabetes mellitus
[ICD-11: 5A11]
|
Phase 3 | [1] | ||
|
Prediabete
[ICD-11: 5A40]
|
Investigative | [2] | |||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. Enhancing Drug Efficacy by This Combination | ||||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Experiment 1 Reporting the Effect of This Combination | [2] | |||||
| In-vivo Model | Alogliptin (0.03%) and voglibose (0.001%) alone or in combination were administered in the diet to prediabetic db/db mice. | |||||
| Experimental
Result(s) |
Chronic treatment with alogliptin in combination with voglibose concurrently increased active GLP-1 circulation, increased insulin secretion, decreased glucagon secretion, prevented the onset of the disease, and preserved pancreatic beta-cells and islet structure in prediabetic db/db mice. | |||||

