Drug Details
| General Information of the Drug (ID: DR4332) | ||||
|---|---|---|---|---|
| Name |
Chlorhexidine
|
|||
| Synonyms |
chlorhexidine; 55-56-1; Rotersept; Fimeil; Hexadol; Soretol; Chlorhexidin; Chlorhexidinum; Chlorohexidine; Nolvasan; Cloresidina [DCIT]; Chlorhexidin [Czech]; Chlorhexidinum [INN-Latin]; Clorhexidina [INN-Spanish]; Hibistat; 1,6-Bis(p-chlorophenyldiguanido)hexane; 1,6-Di(4'-chlorophenyldiguanido)hexane; UNII-R4KO0DY52L; 1,6-Bis(5-(p-chlorophenyl)biguandino)hexane; Exidine; Tubulicid; 1,1'-Hexamethylenebis(5-(p-chlorophenyl)biguanide); 1,1'-Hexamethylene bis(5-(p-chlorophenyl)biguanide); 2,4,11,13-Tetraazatetradecanediimidamide, N,N''-bis(4-chlorophenyl)-3,12-diimino-; Sterilon; CHEMBL790; R4KO0DY52L; MLS001332388; CHEBI:3614; Cloresidina; Clorhexidina; 56-95-1; Biguanide, 1,1'-hexamethylenebis(5-(p-chlorophenyl)-; Chlorhexidine, 98%; CAS-55-56-1; NCGC00016246-03; SMR000857146; Sterido; Savlon babycare; N',N'''''-hexane-1,6-diylbis[N-(4-chlorophenyl)(imidodicarbonimidic diamide)]; DSSTox_CID_13314; DSSTox_RID_79062; N,N'-Bis(4-chlorophenyl)-3,12-diimino-2,4,11,13-tetraazatetradecanediimidamide; DSSTox_GSID_33314; Chlorhexidine [INN:BAN]; Chlorhexidine dihydrochloride; MLS001304094; (1E)-2-[6-[[amino-[(E)-[amino-(4-chloroanilino)methylidene]amino]methylidene]amino]hexyl]-1-[amino-(4-chloroanilino)methylidene]guanidine; 1-(4-chlorophenyl)-3-[N-[6-[[N-[N-(4-chlorophenyl)carbamimidoyl]carbamimidoyl]amino]hexyl]carbamimidoyl]guanidine; N-(4-chlorophenyl)-1-3-(6-{N-[3-(4-chlorophenyl)carbamimidamidomethanimidoyl]amino}hexyl)carbamimidamidomethanimidamide; CCRIS 9230; Chlorhexidine (INN); C22H30Cl2N10; HSDB 7196; Merfen-incolore (TN); SR-01000799135; Nolvasan (*Diacetate*); 1,1'-Hexamethylenebis(5-[p-chlorophenyl]biguanide); SMR000718621; EINECS 200-238-7; Lisium (*Dihydrochloride*); BRN 2826432; 1,6-Di(N-p-chlorophenyldiguanido)hexane; Dentisept [veterinary] (TN); 1,6-Bis(N5-[p-chlorophenyl]-N1-biguanido)hexane; Prestwick_53; Chlorhexidine (1); Hibidil (Salt/Mix); Hibisol (Salt/Mix); Chlorhexidine diacetate salt hydrate; Hibitane (Salt/Mix); Hibiscrub (Salt/Mix); Hibispray (Salt/Mix); NSC526936; Spectrum_000237; Savloclens (Salt/Mix); Prestwick0_000143; Prestwick1_000143; Prestwick2_000143; Prestwick3_000143; Spectrum2_000135; Spectrum3_000339; Spectrum4_000277; Spectrum5_001322; chlorhexidine diacetate salt; EC 200-238-7; SCHEMBL3984; Chlorhexidine, >=99.5%; BSPBio_000246; BSPBio_001977; KBioGR_000774; KBioSS_000717; 4-12-00-01201 (Beilstein Handbook Reference); MLS001332387; MLS002154209; DivK1c_000761; SPBio_000210; SPBio_002185; BPBio1_000272; DTXSID2033314; BDBM51937; BDBM64773; cid_9552079; KBio1_000761; KBio2_000717; KBio2_003285; KBio2_005853; KBio3_001197; 2,4,11,13-Tetraazatetradecanediimidamide, N1,N14-bis(4-chlorophenyl)-3,12-diimino-; cid_12303047; NINDS_000761; REGID_for_CID_9552079; BDBM152706; HMS1568M08; HMS2095M08; HMS2233B16; HMS3712M08; HY-B1248; Tox21_110325; Tox21_201404; Tox21_303445; BDBM50170723; s5397; SBB057564; STK089248; AKOS005394319; Tox21_110325_1; CCG-220143; CS-4958; DB00878; EBD2224760; MCULE-4644073142; 2,4,11,13-Tetraazatetradecanediimidamide, N,N'-bis(4-chlorophenyl)-3,12-diimino-; IDI1_000761; N,N''''-hexane-1,6-diylbis[N'-(4-chlorophenyl)(imidodicarbonimidic diamide)]; N,N'-Bis(4-chlorophenyl)-3,12-diimino-2,4,11,13-tetraazatetradeca- nediimidamide; QTL1_000020; NCGC00016246-01; NCGC00016246-02; NCGC00016246-04; NCGC00016246-05; NCGC00016246-06; NCGC00016246-07; NCGC00016246-09; NCGC00016246-13; NCGC00091025-01; NCGC00091025-02; NCGC00091025-04; NCGC00247766-01; NCGC00257242-01; NCGC00258955-01; (1E)-2-[6-[[amino-[(E)-[amino-(4-chloroanilino)methylene]amino]methylene]amino]hexyl]-1-[amino-(4-chloroanilino)methylene]guanidine; AS-12648; Chlorhexidine, purum, >=99.0% (HPLC); SBI-0051301.P003; AB00053427; C06902; D07668; AB00053427-24; AB00053427-28; AB00053427_29; 009C673; Q-200828; SR-01000799135-5; 1,1''-Hexamethylene bis(5-(p-chlorophenyl)biguanide); BRD-K52256627-300-03-3; BRD-K52256627-300-05-8; SR-01000799135-10; SR-01000799135-11; Chlorhexidine, European Pharmacopoeia (EP) Reference Standard; 1,1'-(Hexane-1,6-diyl)bis[5-(4-chlorophenyl)biguanide] diacetate; Chlorhexidine, United States Pharmacopeia (USP) Reference Standard; Chlorhexidine, Pharmaceutical Secondary Standard; Certified Reference Material; N'',N''''''''''-hexane-1,6-diylbis[N-(4-chlorophenyl)(imidodicarbonimidic diamide)]; N,N''-Bis(4-chlorophenyl)-3,12-diimino-2,4,11,13-tetraazatetradecanediimidamide; (1E)-2-[6-[[amino-[(E)-[amino-(4-chloroanilino)methylene]amino]methylene]amino]hexyl]-1-[amino-(4-chloroanilino)methylene]guanidine;hydrochloride; (1E)-2-[6-[[amino-[(E)-[amino-(4-chloroanilino)methylidene]amino]methylidene]amino]hexyl]-1-[amino-(4-chloroanilino)methylidene]guanidine;hydrochloride; (1E)-2-[6-[[azanyl-[(E)-[azanyl-[(4-chlorophenyl)amino]methylidene]amino]methylidene]amino]hexyl]-1-[azanyl-[(4-chlorophenyl)amino]methylidene]guanidine; (1E)-2-[6-[[azanyl-[(E)-[azanyl-[(4-chlorophenyl)amino]methylidene]amino]methylidene]amino]hexyl]-1-[azanyl-[(4-chlorophenyl)amino]methylidene]guanidine;hydrochloride; {[(4-chlorophenyl)amino]iminomethyl}{[(6-{[({[(4-chlorophenyl)amino]iminomethy l}amino)iminomethyl]amino}hexyl)amino]iminomethyl}amine; 2-[amino-[6-[[amino-[(E)-[amino-(4-chloroanilino)methylidene]amino]methylidene]amino]hexylimino]methyl]-1-(4-chlorophenyl)guanidine; 2-[amino-[6-[[amino-[(E)-[amino-(4-chloroanilino)methylidene]amino]methylidene]amino]hexylimino]methyl]-1-(4-chlorophenyl)guanidine;hydrochloride
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Bacterial infection [ICD-11: 1A00-1C4Z] | Approved | [1] | |
| Structure |
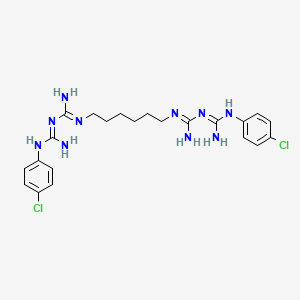
|
Click to Download Mol2D MOL |
||
| ADMET Property |
Absorption Tmax
The time to maximum plasma concentration (Tmax) is 30 min
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 3: high solubility and low permeability
Elimination
Excretion of chlorhexidine gluconate occurs almost exclusively via the feces, with less than 1% of an ingested dose excreted in the urine
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C22H30Cl2N10
|
|||
| PubChem CID | ||||
| Canonical SMILES |
C1=CC(=CC=C1NC(=NC(=NCCCCCCN=C(N)N=C(N)NC2=CC=C(C=C2)Cl)N)N)Cl
|
|||
| InChI |
1S/C22H30Cl2N10/c23-15-5-9-17(10-6-15)31-21(27)33-19(25)29-13-3-1-2-4-14-30-20(26)34-22(28)32-18-11-7-16(24)8-12-18/h5-12H,1-4,13-14H2,(H5,25,27,29,31,33)(H5,26,28,30,32,34)
|
|||
| InChIKey |
GHXZTYHSJHQHIJ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 55-56-1
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Xylitol | Saccharomyces cerevisiae | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Experimental
Result(s) |
The XYL + CHX combination was efficient and superior to single treatments in controlling biofilm and suppressing S. mutans. | |||||
| 1,8-cineole + 1,8-cineole | Click to Show/Hide the Molecular Data of This NP | |||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [3] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vitro Model | Staphylococcus aureus | Microorganism model | Staphylococcus aureus | |||
| Pseudomonas aeruginosa | Microorganism model | Pseudomonas aeruginosa | ||||
| Escherichia coli | Microorganism model | Escherichia coli | ||||
| Candida albicans | Microorganism model | Candida albicans | ||||
| Experimental
Result(s) |
CHG may be combined with either crude EO or its major component 1,8-cineole for enhanced, synergistic antimicrobial activity against a wide range of microorganisms in planktonic and biofilm modes of growth. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Bacterial Dihydropteroate synthetase (Bact folP) | Molecule Info | [4] | |

