Drug Details
| General Information of the Drug (ID: DR5795) | ||||
|---|---|---|---|---|
| Name |
Verapamil
|
|||
| Synonyms |
VERAPAMIL; 52-53-9; Iproveratril; Vasolan; Dilacoran; Isoptin; Falicard; Finoptin; Isoptine; Calan; Lekoptin; Verapamilo; Verapamilum; Isotopin; Securon; Verelan; Calcan; Tarka; D-365; Cardibeltin; Izoptin; CP-16533-1; Covera-HS; Verpamil; Verelan PM; 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-(propan-2-yl)pentanenitrile; 5-((3,4-Dimethoxyphenethyl)methylamino)-2-(3,4-dimethoxyphenyl)-2-isopropylvaleronitrile; Cardiagutt; Berkatens; Dignover; Geangin; Veramex; Quasar; Univer; 5-((3,4-dimethoxyphenethyl)(methyl)amino)-2-(3,4-dimethoxyphenyl)-2-isopropylpentanenitrile; Novo-Veramil; Apo-Verap; Isoptin SR; Nu-Verap; Calan SR; Isoptimo; Benzeneacetonitrile, alpha-(3-((2-(3,4-dimethoxyphenyl)ethyl)methylamino)propyl)-3,4-dimethoxy-alpha-(1-methylethyl)-; (+/-)-Verapamil hydrochlorid; CHEBI:77733; Cardiabeltin; Cardioprotect; Durasoptin; Hexasoptin; Veratensin; Veroptinstada; Calaptin; Caveril; Civicor; Coraver; Corpamil; Harteze; Hormitol; Ikapress; Inselon; Isoptino; Jenapamil; Lodixal; Magotiron; Praecicor; Robatelan; Vasomil; Vasopten; Verabeta; Veracor; Verahexal; Veraloc; Veramil; Verapin; Verasal; Verasifar; Verdilac; Vetrimil; Akilen; Elthon; Flamon; Ikacor; Univex; Vortac; Anpec; Ormil; Rapam; Civicor Retard; Isoptin Retard; Manidon Retard; Valeronitrile, 5-((3,4-dimethoxyphenethyl)methylamino)-2-(3,4-dimethoxyphenyl)-2-isopropyl-; Vera-Sanorania; Verapamil Acis; Verapamil Atid; Verapamil Ebewe; Verapamil Riker; Verapamil Verla; (+/-)-Verapamil;CP-16533-1; Benzeneacetonitrile, .alpha.-[3-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]propyl]-3,4-dimethoxy-.alpha.-(1-methylethyl)-; Verapamil Basics; Verapamil Nordic; Verapamil-AbZ; Novapamyl LP; Verapamil AL; Verapamil NM; Verapamil PB; Verapamil SR; Cordilox SR; Dilacoran HTA; Veracaps SR; Verapamil MSD; Verapamilum [INN-Latin]; Arpamyl LP; Hexasoptin Retard; Verapamil Henning; Verelan SR; NCGC00016083-09; Verapamilo [INN-Spanish]; 2-(3,4-dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methylamino]-2-propan-2-ylpentanenitrile; Verapamil Injection; CP-16,533-1; Verapamil NM Pharma; Calaptin 240 SR; Verapress 240 SR; 5-[(3,4-Dimethoxyphenethyl)methylamino]-2-(3,4-dimethoxyphenyl)-2-isopropylvaleronitrile; DSSTox_CID_21152; DSSTox_RID_79636; DSSTox_GSID_41152; (+/-)-VERAPAMIL; Verapamil [USAN:INN:BAN]; dl-Verapamil; r,s-verapamil; CAS-52-53-9; CCRIS 6749; NSC272366; Verapamil (USAN/INN); EINECS 200-145-1; Ansyr; NSC 272306NA; EINECS 260-462-6; Calan (Salt/Mix); Akilen (Salt/Mix); alpha-((N-Methyl-N-homoveratryl)-gamma-aminopropyl)-3,4-dimethoxyphenylacetonitrile; Isoptin (Salt/Mix); alpha-(3-((2-(3,4-Dimethoxyphenyl)ethyl)-methylamino)propyl)-3,4-dimethoxy-alpha-(1-methylethyl)benzeneacetonitrile; alpha-Isopropyl-alpha-((N-methyl-N-homoveratryl)-gamma-aminopropyl)-3,4-dimethoxyphenylacetonitrile; Cordilox (Salt/Mix); delta-365; Covera-HS (Salt/Mix); Prestwick0_000141; Prestwick1_000141; Prestwick2_000141; Prestwick3_000141; Spectrum2_001740; Spectrum4_000906; Spectrum5_001786; CP 16533-1; D 365; CHEMBL6966; Lopac0_001237; SCHEMBL16742; BSPBio_000242; BSPBio_001513; BSPBio_002358; KBioGR_000233; KBioGR_001372; KBioGR_002343; KBioSS_000233; KBioSS_002346; 56949-77-0; Benzeneacetonitrile, alpha-(3-((2-(3,4-dimethoxyphenyl)ethyl)methylamino) propyl)-3,4-dimethoxy-alpha-(1-methylethyl)-; MLS006011414; DivK1c_000399; SPBio_001820; SPBio_002181; BPBio1_000268; GTPL2406; CP 16533-1 (Verapamil); DTXSID9041152; SCHEMBL13287282; (A+/-)-Verapamil hydrochloride; BDBM81939; HSDB 3928; KBio1_000399; KBio2_000233; KBio2_002343; KBio2_002801; KBio2_004911; KBio2_005369; KBio2_007479; KBio3_000465; KBio3_000466; KBio3_002823; cMAP_000023; NINDS_000399; NSC-272306NA; Bio1_000425; Bio1_000914; Bio1_001403; Bio2_000233; Bio2_000713; HMS1791L15; HMS1989L15; HMS2089H17; HMS3402L15; Tox21_110300; NSC_62969; STK538085; AKOS005468962; Tox21_110300_1; CCG-205311; DB00661; MCULE-3016077278; SDCCGSBI-0051204.P005; CAS_52-53-9; IDI1_000399; IDI1_033983; NCGC00016083-04; NCGC00016083-05; NCGC00016083-06; NCGC00016083-07; NCGC00016083-08; NCGC00016083-10; NCGC00016083-11; NCGC00016083-13; NCGC00016083-14; NCGC00016083-15; NCGC00016083-16; NCGC00016083-17; NCGC00016083-18; NCGC00016083-20; NCGC00016083-25; NCGC00016083-34; NCGC00024710-04; NCGC00024710-05; NCGC00024710-06; NCGC00024710-07; NCGC00024710-08; NCGC00024710-09; NCGC00344584-01; 2-(3,4-Dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methyl-amino]-2-(1-methylethyl) pentanenitrile; 2-(3,4-dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methyl-amino]-2-isopropyl-pentanenitrile; AC-16016; BP-21223; HY-14275; NCI60_020143; SMR001550201; SBI-0051204.P003; CP-165331; AB00053495; CS-0002967; FT-0603225; FT-0675801; ST50826367; 1394-EP2270011A1; 1394-EP2272841A1; 1394-EP2272972A1; 1394-EP2272973A1; 1394-EP2275420A1; 1394-EP2277865A1; 1394-EP2277872A1; 1394-EP2280008A2; 1394-EP2298742A1; 1394-EP2298764A1; 1394-EP2298765A1; 1394-EP2298776A1; 1394-EP2298779A1; 1394-EP2301923A1; 1394-EP2301931A1; 1394-EP2301936A1; 1394-EP2305648A1; 1394-EP2314585A1; 1394-EP2316832A1; 1394-EP2316833A1; 52V114; C07188; D02356; AB00053495-20; AB00053495_21; L001330; Q410291; BRD-A09533288-001-02-7; BRD-A09533288-003-05-6; CP-165331 / CP-16533-1; F2173-0851; VERAPAMIL, Dexverapamil, Verapamyl hydrochloride, VERAPAMIL HYDROCHLORIDE; .alpha.-((N-Methyl-N-homoveratryl)-.gamma.-aminopropyl)-3,4-dimethoxyphenylacetonitrile; (?)-alpha-[3-[[2-(3,4-Dimethoxyphenyl)ethyl](methyl)amino]propyl]-3,4-dimethoxy-alpha-(1-methylethyl)benzeneacetonitrile; 2-(3,4-Dimethoxyphenyl)-5-((2-(3,4-dimethoxyphenyl)ethyl)(methyl)amino)-2-isopropylpentanenitrile, (+/-)-; 2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino]-2-isopropylpentanenitrile #; 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl]methylamino}-2-(methy lethyl)pentanenitrile
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Hypertension [ICD-11: BA00] | Approved | [1] | |
| Structure |
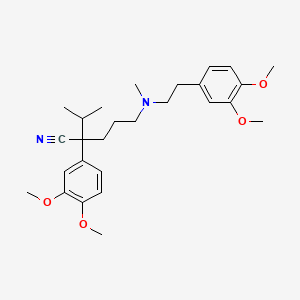
|
Click to Download Mol2D MOL |
||
| ADMET Property |
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 1: high solubility and high permeability
Clearance
The apparent oral clearance of drug is 1007 mL/min
Clearance
The drug present in the plasma can be removed from the body at the rate of 18 mL/min/kg
Elimination
Approximately 70% of an administered dose is excreted as metabolites in the urine and more than 16% in the feces within 5 days
Elimination
1.5% of drug is excreted from urine in the unchanged form
Half-life
The concentration or amount of drug in body reduced by one-half in 2.8 - 7.4 hours
Half-life
The concentration or amount of drug in body reduced by one-half in 2.8 hours
Metabolism
The drug is metabolized via the liver
Unbound Fraction
The unbound fraction of drug in plasma is 0.093%
Vd
The volume of distribution (Vd) of drug is 300 L
Vd
Fluid volume that would be required to contain the amount of drug present in the body at the same concentration as in the plasma 3.7 L/kg
Water Solubility
The ability of drug to dissolve in water is measured as 0.75 mg/mL
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C27H38N2O4
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CC(C)C(CCCN(C)CCC1=CC(=C(C=C1)OC)OC)(C#N)C2=CC(=C(C=C2)OC)OC
|
|||
| InChI |
1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3
|
|||
| InChIKey |
SGTNSNPWRIOYBX-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 52-53-9
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Trandolapril | Homo sapiens | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Clinical trial | |||||
| Experimental
Result(s) |
A fixed-dose combination of trandolapril-verapamil seems to be an effective and safe option for the management of stage 2 hypertension in Mexican patients uncontrolled by monotherapy. | |||||
| β. A List of Natural Product(s) Able to Decrease the Adverse Effect of This Drug | ||||||
| Reserpine | Rauvolfia serpentina | Click to Show/Hide the Molecular Data of This NP | ||||
| Decreasing Adverse Drug Reaction | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [3] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Molecule(s)
Regulation |
Up-regulation | Expression | CD80 | Molecule Info |
Pathway MAP
|
|
| Up-regulation | Expression | HLA-DRB5 | Molecule Info |
Pathway MAP
|
||
| Up-regulation | Expression | KLRK1 | Molecule Info |
Pathway MAP
|
||
| In-vitro Model | YAC-1 | CVCL_2244 | Mouse lymphoma | Mus musculus | ||
| B16-F10 | CVCL_0159 | Mouse melanoma | Mus musculus | |||
| In-vivo Model | For a xenograft model, Female C57BL/6 mice, 6-8 weeks of age, 18-20 g, were subcutaneously (s.c.) immunized in the abdominal region with 2x105, 5x105 or 1x106 B16F10 cells. | |||||
| Experimental
Result(s) |
The B16F10 tumor cell vaccine treated with MIP in combination with RP and VP was safe and efficient. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Calcium channel alpha-1G (CACNA1G) | Molecule Info | [4] | |
| KEGG Pathway | MAPK signaling pathway | Click to Show/Hide | ||
| 2 | Calcium signaling pathway | |||
| 3 | Circadian entrainment | |||
| 4 | Type II diabetes mellitus | |||
| Panther Pathway | Endogenous cannabinoid signaling | Click to Show/Hide | ||
| 2 | GABA-B receptor II signaling | |||
| 3 | Nicotine pharmacodynamics pathway | |||
| Pathwhiz Pathway | Muscle/Heart Contraction | Click to Show/Hide | ||
| Pathway Interaction Database | Regulation of nuclear beta catenin signaling and target gene transcription | Click to Show/Hide | ||
| Reactome | NCAM1 interactions | Click to Show/Hide | ||
| WikiPathways | NCAM signaling for neurite out-growth | Click to Show/Hide | ||
| 2 | Nicotine Activity on Chromaffin Cells | |||