Drug Details
| General Information of the Drug (ID: DR8289) | ||||
|---|---|---|---|---|
| Name |
Acyclovir
|
|||
| Synonyms |
acyclovir; Aciclovir; Acycloguanosine; 59277-89-3; Zovirax; Vipral; Virorax; Wellcome-248U; 9-[(2-Hydroxyethoxy)methyl]guanine; Aciclovirum; Zovir; 2-Amino-9-((2-hydroxyethoxy)methyl)-1H-purin-6(9H)-one; Sitavig; 9-(2-Hydroxyethoxy)methylguanine; 9-HYROXYETHOXYMETHYLGUANINE; W-248-U; Acyclovir-side chain-2-3H; 9-((2-Hydroxyethoxy)methyl)guanine; UNII-X4HES1O11F; 2-Amino-9-[(2-hydroxyethoxy)methyl]-1,9-dihydro-6H-purin-6-one; Acyclovir-d4; NSC 645011; CHEBI:2453; 2-amino-9-(2-hydroxyethoxymethyl)-3H-purin-6-one; Acyclovir (Aciclovir); 2-Amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-6H-purin-6-one; 6H-Purin-6-one, 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-; Zovirax (TN); MFCD00057880; 9-[(2-Hydroxyethoxy)-methyl]guanine; 2-Amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; X4HES1O11F; MLS000069633; 141294-79-3; Activir; 9-(2-Hydroxyethoxymethyl)guanine; 6H-Purin-6-one, 2-amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-; 2-amino-9-(2-hydroxyethoxymethyl)-1H-purin-6-one; NSC645011; NSC-645011; AC2; 2-amino-9-((2-hydroxyethoxy)methyl)-3H-purin-6(9H)-one; Acycloguanosine, 98%; NCGC00015061-02; Aciclovirum [Latin]; Aciclovier; Hascovir; SMR000058225; Genvir; Maynar; Zyclir; 2-amino-9-[(2-hydroxyethoxy)methyl]-6,9-dihydro-3H-purin-6-one; CAS-59277-89-3; 6H-Purin-6-one, 1,9-dihydro-2-amino-9-((2-hydroxyethoxy)methyl)-; DSSTox_CID_2556; DSSTox_RID_76626; Aciclovirum [INN-Latin]; DSSTox_GSID_22556; 2-amino-9-[(2-hydroxyethoxy)methyl]hydropurin-6-one; Acicloftal; Cargosil; Viropump; AcycloFoam; 2-amino-9-[(2-hydroxyethoxy)methyl]-3,9-dihydro-6H-purin-6-one; Acic; BW-248U; 2-amino-9-{[(2-hydroxyethyl)oxy]methyl}-1,9-dihydro-6H-purin-6-one; Acyclo-V; Acyclovir Lauriad; DRG-0008; Acyclovir (USP); BW 248U; CCRIS 1953; 1185179-33-2; HSDB 6511; SR-01000075540; Acyclovir [USAN:USP]; EINECS 261-685-1; ACV & Pluronic F-68; Acyclovir & Pluronic F-68; Cyclovir; Poviral; Sitavir; 2-amino-9-((2-hydroxyethoxy)methyl)-3,9-dihydro-6H-purin-6-one; Prestwick_6; ACYCLOVIR-SIDECHAIN-2-3H; 1pwy; Aciclovir [INN]; BW-248-U; Sitavig (TN); PubChem9572; Spectrum_001739; Opera_ID_1674; Prestwick0_000086; Prestwick1_000086; Prestwick2_000086; Prestwick3_000086; Spectrum2_001563; Spectrum3_001874; Spectrum4_000225; Spectrum5_001093; Lopac-A-4669; Aciclovir (JP17/INN); CHEMBL184; A 4669; Acycloguanosine (Acyclovir); SCHEMBL3175; Lopac0_000037; BSPBio_000012; BSPBio_003348; KBioGR_000889; KBioSS_002219; ARONIS27002; BIDD:GT0646; DivK1c_000185; SPECTRUM1503603; SPBio_001466; SPBio_001951; BPBio1_000014; GTPL4829; SCHEMBL9828560; 9(2-hydroxyethoxymethyl)guanine; DTXSID1022556; HMS500J07; KBio1_000185; KBio2_002219; KBio2_004787; KBio2_007355; KBio3_002850; NINDS_000185; HMS1568A14; HMS1922E08; HMS2090G09; HMS2095A14; HMS2234K21; HMS3259N10; HMS3260G15; HMS3269M15; HMS3372K02; HMS3413D21; HMS3655C14; HMS3677D21; HMS3712A14; Pharmakon1600-01503603; BCP11036; EBD48195; ZINC1530555; 9-(2-hydroxyethoxy methyl) guanine; Tox21_110075; Tox21_500037; 59277-89-3 (free); 69657-51-8 (Na salt); ANW-43941; BBL009642; BDBM50021776; BDBM50103518; CCG-39909; NSC758477; NSC780378; s1807; SBB063281; STK796771; STL257059; STL301862; AKOS000656213; AKOS015995680; AKOS022135433; Tox21_110075_1; AC-8068; CS-1353; DB00787; KS-1027; LP00037; MCULE-2703274259; NC00717; NSC-758477; NSC-780378; SDCCGSBI-0050026.P003; IDI1_000185; SMP1_000007; NCGC00015061-01; NCGC00015061-03; NCGC00015061-04; NCGC00015061-05; NCGC00015061-06; NCGC00015061-07; NCGC00015061-08; NCGC00015061-09; NCGC00015061-10; NCGC00015061-12; NCGC00015061-13; NCGC00015061-28; NCGC00022426-03; NCGC00093555-01; NCGC00093555-02; NCGC00093555-03; NCGC00093555-04; NCGC00167756-01; NCGC00167756-02; NCGC00260722-01; NCGC00381719-03; AK-24687; HY-17422; ST024744; SY051130; Acycloguanosine, >=99% (HPLC), powder; AB0012294; AB0068584; Aciclovir 1.0 mg/ml in Dimethyl Sulfoxide; AM20100442; EU-0100037; FT-0621607; FT-0657847; SW196324-3; 6383-EP2270005A1; 6383-EP2305243A1; 6383-EP2305640A2; 6383-EP2305808A1; 6383-EP2314582A1; 6383-EP2314585A1; C06810; D00222; J10243; M-1904; 32284-EP2281815A1; 32284-EP2301933A1; 32284-EP2311827A1; 277A893; A832236; Q147101; Q-200591; SR-01000075540-1; SR-01000075540-3; SR-01000075540-5; 2-amino-9-[(2-hydroxyethoxy)methyl]-9H-purin-6-ol; 2-azanyl-9-(2-hydroxyethyloxymethyl)-3H-purin-6-one; BRD-K32318651-001-17-9; Aciclovir, British Pharmacopoeia (BP) Reference Standard; F2173-0946; Aciclovir, European Pharmacopoeia (EP) Reference Standard; 2-Amino-9-(2-hydroxy-ethoxymethyl)-5,9-dihydro-purin-6-one; Acyclovir, United States Pharmacopeia (USP) Reference Standard; 2-Amino-9-(2-hydroxy-ethoxymethyl)-5,9-dihydro-purin-6-one (ACV); 2-Amino-9-[(2-hydroxyethoxy)methyl]-1,9-dihydro-6H-purin-6-one #; 2-amino-9-[(1,1,2,2-tetradeuterio-2-hydroxyethoxy)methyl]-1H-purin-6-one; ACV; Acycloguanosine; Acyclovir; NSC 645011; NSC-645011; NSC645011; Acyclovir, Pharmaceutical Secondary Standard; Certified Reference Material; Aciclovir for peak identification 1, European Pharmacopoeia (EP) Reference Standard; Aciclovir for peak identification 2, European Pharmacopoeia (EP) Reference Standard; Aciclovir for system suitability, European Pharmacopoeia (EP) Reference Standard
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Virus infection [ICD-11: 1D90-1D9Z] | Approved | [1] | |
| Structure |
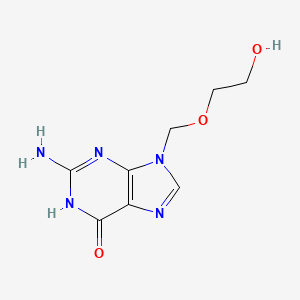
|
Click to Download Mol2D MOL |
||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C8H11N5O3
|
|||
| PubChem CID | ||||
| Canonical SMILES |
C1=NC2=C(N1COCCO)N=C(NC2=O)N
|
|||
| InChI |
1S/C8H11N5O3/c9-8-11-6-5(7(15)12-8)10-3-13(6)4-16-2-1-14/h3,14H,1-2,4H2,(H3,9,11,12,15)
|
|||
| InChIKey |
MKUXAQIIEYXACX-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 59277-89-3
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Betulin | Betula pendula | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vitro Model | Vero | CVCL_0059 | Healthy | Chlorocebus sabaeus | ||
| Experimental
Result(s) |
Strong and moderate synergistic antiviral effects were observed for betulin and ACV against HSV-1 when the concentrations of ACV and betulin were higher than 0.068 and 0.4 microg/ml, respectively. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Herpes simplex virus DNA polymerase UL30 (HSV UL30) | Molecule Info | [3] | |
| Varicella-zoster virus DNA polymerase (VZV ORF28) | Molecule Info | [4] | ||

