Drug Details
| General Information of the Drug (ID: DR9574) | ||||
|---|---|---|---|---|
| Name |
Metronidazole
|
|||
| Synonyms |
metronidazole; 443-48-1; Flagyl; Metronidazol; 2-Methyl-5-nitroimidazole-1-ethanol; Anagiardil; Trichazol; MetroGel; Bayer 5360; Gineflavir; Meronidal; Metronidaz; Novonidazol; Trichopol; Trivazol; Danizol; Mexibol; Vagilen; Clont; 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethanol; Flagemona; Giatricol; Metronidazolo; Sanatrichom; Takimetol; Trichocide; Trichomol; Trikacide; Acromona; Atrivyl; Deflamon; Efloran; Entizol; Flagesol; Monagyl; Monasin; Orvagil; Trichex; Tricocet; Trikamon; Trikojol; Trikozol; Trimeks; Vagimid; Vertisal; Wagitran; Arilin; Bexon; Elyzol; Eumin; Flagil; Klion; Klont; Nalox; Tricom; neo-Tric; Tricowas B; Deflamon-wirkstoff; Protostat; Satric; MetroCream; MetroLotion; MetroGel-Vaginal; CONT; NIDA; Methronidazole; Metromidol; Trichopal; Flegyl; Fossyol; 1H-Imidazole-1-ethanol, 2-methyl-5-nitro-; Flagyl Er; Metronidazolum; Metro I.V.; Metrolyl; Metric 21; Trichomonacid 'pharmachim'; 1-(2-Hydroxyethyl)-2-methyl-5-nitroimidazole; 2-(2-methyl-5-nitroimidazol-1-yl)ethanol; RP 8823; NSC-50364; Metronidazole in Plastic Container; 2-Methyl-1-(2-hydroxyethyl)-5-nitroimidazole; 2-Methyl-3-(2-hydroxyethyl)-4-nitroimidazole; SC 10295; MFCD00009750; 1-(beta-Ethylol)-2-methyl-5-nitro-3-azapyrrole; 1-(2-Hydroxy-1-ethyl)-2-methyl-5-nitroimidazole; 1-(beta-Hydroxyethyl)-2-methyl-5-nitroimidazole; 1-Hydroxyethyl-2-methyl-5-nitroimidazole; Imidazole-1-ethanol, 2-methyl-5-nitro-; 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethan-1-ol; FLAGYL I.V. RTU IN PLASTIC CONTAINER; 1-(beta-Oxyethyl)-2-methyl-5-nitroimidazole; NSC 50364; UNII-140QMO216E; 2-(2-methyl-5-nitro-1-imidazolyl)ethanol; 2-(2-methyl-5-nitro-imidazol-1-yl)ethanol; BAY-5360; NSC69587; Noritate; MLS000028590; CHEBI:6909; 140QMO216E; Metro Gel; NSC50364; NSC-69587; Metronidazole, 99%; NCGC00016446-06; CAS-443-48-1; Metrolag; Metrotop; Rathimed; SMR000058175; Vandazole; Zadstat; 2-(2-methyl-5-nitroimidazolyl)ethan-1-ol; Tricho cordes; DSSTox_CID_892; Metronidazolo [DCIT]; Tricho-gynaedron; DSSTox_RID_75848; DSSTox_GSID_20892; Mexibol 'silanes'; 99616-64-5; Metro I.V. In Plastic Container; 1-(.beta.-Ethylol)-2-methyl-5-nitro-3-azapyrrole; 1-(.beta.-Hydroxyethyl)-2-methyl-5-nitroimidazole; Metronidazol [INN-Spanish]; Metronidazolum [INN-Latin]; Metronidazole-13C2,15N2; Flagyl I.V. RTU; Flagyl 375; Trichobrol; Florazole; Mepagyl; Nidagyl; Rosased; Zidoval; Caswell No. 579AA; WLN: T5N CNJ A2Q B1 ENW; Noritate (TN); CCRIS 410; Metro cream & gel; Flagyl (TN); HSDB 3129; WLN: T6NTJ DQ ANU1- ET5N CNJ A1 BNW; SR-01000000244; EINECS 207-136-1; NSC 69587; EPA Pesticide Chemical Code 120401; BRN 0611683; Polibiotic; Trikhopol; Donnan; Flazol; CB-01-14 MMX; Metro IV; Vandazole (TN); Metronidazole,(S); Prestwick_334; Nuvessa (TN); IDR-90105; Cimetrol 500LPCI; Metronidazole solution; RP-8823; Metronidazole, BioXtra; Metronidazole (Flagyl); PubChem15970; Spectrum_001035; Metronidazole [USAN:USP:INN:BAN:JAN]; ACMC-209jxc; HELIDAC (Salt/Mix); Maybridge1_001999; Opera_ID_1585; Prestwick0_000081; Prestwick1_000081; Prestwick2_000081; Prestwick3_000081; Spectrum2_000883; Spectrum3_000506; Spectrum4_000060; Spectrum5_001289; M0924; CHEMBL137; NCIOpen2_000337; SCHEMBL23042; BSPBio_000002; BSPBio_002031; KBioGR_000559; KBioSS_001515; 5-23-05-00063 (Beilstein Handbook Reference); MLS000758286; MLS001424018; ARONIS24285; BIDD:GT0107; DivK1c_000007; SPECTRUM1500412; SPBio_000666; SPBio_001941; BAYER-5360; BPBio1_000004; DTXSID2020892; Flagyl I.V. RTU (Salt/Mix); BCBcMAP01_000184; GTPL10914; HMS500A09; HMS547C19; KBio1_000007; KBio2_001515; KBio2_004083; KBio2_006651; KBio3_001531; Metronidazole (JP17/USP/INN); Metronidazole, analytical standard; NINDS_000007; HMS1568A04; HMS1920N19; HMS2051G07; HMS2090B19; HMS2091F14; HMS2095A04; HMS2231E11; HMS3373O05; HMS3393G07; HMS3655E22; HMS3712A04; Pharmakon1600-01500412; ZINC113442; BCP13757; HY-B0318; Tox21_110441; Tox21_202413; Tox21_302794; ANW-30094; BBL005452; BDBM50375309; CCG-40016; FP-250; NSC757118; s1907; SBB001486; SBB041018; STK177359; Metronidazole 2.0 mg/ml in Methanol; AKOS000269646; AKOS005169650; Tox21_110441_1; DB00916; KS-5140; MCULE-6891596695; NC00020; NSC-757118; 1-(2-Hydroxyethyl)-2-methyl-5-nitroimidazole N-(5-carboxy-5-aminopentane)carbamate; IDI1_000007; SMP1_000189; NCGC00016446-01; NCGC00016446-02; NCGC00016446-03; NCGC00016446-04; NCGC00016446-05; NCGC00016446-07; NCGC00016446-08; NCGC00016446-09; NCGC00016446-11; NCGC00016446-12; NCGC00016446-17; NCGC00022059-03; NCGC00022059-04; NCGC00022059-05; NCGC00256513-01; NCGC00259962-01; AC-23968; AK-77146; ST024769; SY002821; SBI-0051447.P003; AB0008438; DB-051212; Metronidazole, SAJ first grade, >=99.0%; AB00052046; BB 0218386; FT-0603394; SW196613-4; C07203; D00409; M-2794; 74568-EP2275420A1; 74568-EP2292612A2; 74568-EP2305640A2; 74568-EP2305662A1; 74568-EP2308857A1; AB00052046-17; AB00052046_18; AB00052046_19; A826552; Metronidazole, VETRANAL(TM), analytical standard; Q169569; 2-(2-methyl-5-nitro-1H-imidazol-1-yl)-1-ethanol; 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethanol #; Metronidazole, Antibiotic for Culture Media Use Only; Q-201403; SR-01000000244-4; SR-01000000244-5; BRD-K52020312-001-05-2; BRD-K52020312-001-15-1; Z87001124; F1773-0073; Metronidazole, certified reference material, TraceCERT(R); Metronidazole, British Pharmacopoeia (BP) Reference Standard; Metronidazole, European Pharmacopoeia (EP) Reference Standard; Metronidazole, United States Pharmacopeia (USP) Reference Standard; Metronidazole solution, 2.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Metronidazole, Pharmaceutical Secondary Standard; Certified Reference Material
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Amoebiasis [ICD-11: 1A36] | Approved | [1] | |
| Structure |
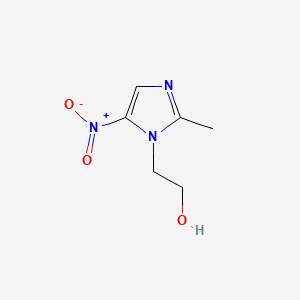
|
Click to Download Mol2D MOL |
||
| ADMET Property |
Absorption Cmax
The maximum plasma concentration (Cmax) of drug is 30-40 mg/L
Absorption Tmax
The time to maximum plasma concentration (Tmax) is 1 h
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 1: high solubility and high permeability
Bioavailability
99% of drug becomes completely available to its intended biological destination(s)
Clearance
The total clearance of drug is 2.1-6.4 L/h/kg
Elimination
Metronidazole and its metabolites are 60 to 80% eliminated in the urine, and 6-15% excreted in the feces
Half-life
The concentration or amount of drug in body reduced by one-half in 6 - 10 h
Metabolism
The drug is metabolized via the hepatic
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 194.55829 micromolar/kg/day
Unbound Fraction
The unbound fraction of drug in plasma is 0.96%
Vd
The volume of distribution (Vd) of drug is 0.51-1.1 L/kg
Water Solubility
The ability of drug to dissolve in water is measured as 10 mg/mL
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C6H9N3O3
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CC1=NC=C(N1CCO)[N+](=O)[O-]
|
|||
| InChI |
1S/C6H9N3O3/c1-5-7-4-6(9(11)12)8(5)2-3-10/h4,10H,2-3H2,1H3
|
|||
| InChIKey |
VAOCPAMSLUNLGC-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 443-48-1
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Green tea catechins | Theaceae | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Molecule(s)
Regulation |
Up-regulation | Expression | HTRA1 | Molecule Info | ||
| In-vitro Model | Porphyromonas gingivalis | Microorganism model | Porphyromonas gingivalis | |||
| Experimental
Result(s) |
Green tea catechins potentiate the effect of antibiotics and modulate adherence and gene expression in Porphyromonas gingivalis. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Bacterial Deoxyribonucleic acid (Bact DNA) | Molecule Info | [3] | |

