Natural Product (NP) Details
| General Information of the NP (ID: NP3975) | |||||
|---|---|---|---|---|---|
| Name |
Cilostazol
|
||||
| Synonyms |
cilostazol; 73963-72-1; Pletal; Cilostazole; Pletaal; OPC-13013; Cilostazolum; Cilostazolum [INN-Latin]; OPC 13013; OPC 21; OPC-21; 6-[4-(1-cyclohexyltetrazol-5-yl)butoxy]-3,4-dihydro-1H-quinolin-2-one; C20H27N5O2; 6-(4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy)-3,4-dihydro-2(1H)-quinolinone; UNII-N7Z035406B; CHEBI:31401; 3,4-Dihydro-6-(4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy)-2(1H)-quinolinone; 6-(4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one; 6-(4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy)-3,4-dihydrocarbostyril; 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydroquinolin-2(1H)-one; MLS000028470; 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-2(1H)-quinolinone; MFCD00866780; N7Z035406B; NCGC00015207-07; SMR000058428; 6-[4-(1-cyclohexyl-1H-1,2,3,4-tetrazol-5-yl)butoxy]-1,2,3,4-tetrahydroquinolin-2-one; DSSTox_CID_25132; DSSTox_RID_80693; DSSTox_GSID_45132; CAS-73963-72-1; Pletal (TN); SR-01000003107; BRN 3632107; Cilostazol,(S); Cilostazol [USAN:USP:INN:BAN:JAN]; Tocris-1692; Opera_ID_488; Spectrum2_001118; Spectrum3_001170; Spectrum4_000772; Spectrum5_001762; Lopac-C-0737; CHEMBL799; C 0737; Lopac0_000218; REGID_for_CID_2754; SCHEMBL16128; BSPBio_002759; KBioGR_001184; MLS000758281; MLS000759507; MLS001076067; MLS002153891; SPECTRUM1505230; SPBio_001256; Cilostazol (JP17/USP/INN); GTPL7148; DTXSID9045132; HSDB 8312; KBio3_002259; BCPP000279; HMS1922N15; HMS2093M14; HMS2096F16; HMS2234C06; HMS3260L17; HMS3268O09; HMS3412B18; HMS3654J13; HMS3676B18; HMS3713F16; Pharmakon1600-01505230; ACT02663; BCP03724; ZINC1552174; Tox21_110098; Tox21_500218; BDBM50225508; CCG-39646; KM1582; NSC758936; s1294; AKOS015855512; Cilostazol, >=98% (HPLC), powder; OPC 13013; OPC 21; Pletaal; Tox21_110098_1; AC-4334; AM90304; BCP9000530; CS-1759; DB01166; KS-5154; LP00218; MCULE-8893820969; NSC 758936; NSC-758936; SDCCGSBI-0050206.P003; 2(1H)-Quinolinone, 3,4-dihydro-6-(4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy)-; 2(1H)-Quionlinone, 6-(4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy)-3,4-dihydro-; 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)-butoxy]-3,4-dihydro-2(1H)-quinolinone; NCGC00015207-01; NCGC00015207-02; NCGC00015207-03; NCGC00015207-04; NCGC00015207-05; NCGC00015207-06; NCGC00015207-08; NCGC00015207-09; NCGC00015207-10; NCGC00015207-11; NCGC00015207-12; NCGC00015207-25; NCGC00022153-02; NCGC00022153-04; NCGC00022153-05; NCGC00022153-06; NCGC00022153-07; NCGC00260903-01; AK111532; HY-17464; BCP0726000145; RETAL;PLETAL;OPC 21;PLETAAL;Cilostal; SBI-0050206.P002; AB0012441; EU-0100218; FT-0602474; FT-0645036; FT-0665038; SW199053-2; D01896; J90029; AB00382988-14; AB00382988_15; AB00382988_16; 963C721; A837982; Q258591; Q-200854; SR-01000003107-2; SR-01000003107-4; SR-01000003107-7; BRD-K67017579-001-04-2; BRD-K67017579-001-05-9; BRD-K67017579-001-07-5; BRD-K67017579-001-13-3; BRD-K67017579-001-17-4; SR-01000003107-10; Cilastatin sodium, Antibiotic for Culture Media Use Only; Cilostazol, United States Pharmacopeia (USP) Reference Standard; 6-[4-(1-cyclohexyl-1,2,3,4-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril; 6-[4-(1-cyclohexyl-5-tetrazolyl)butoxy]-3,4-dihydro-1H-quinolin-2-one; 6-[4-(l-cyclohexyl-1,2,3,4-tetrazol-5-yl)butoxyl]-3,4-dihydrocarbostyril; Cilostazol, Pharmaceutical Secondary Standard; Certified Reference Material; 2(1H)-Quinolinone, 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-; 6-[4-(1-cyclohexyl-1,2,3,4-tetrazol-5-yl)butoxy]-3,4-dihydro-1H-quinolin-2-one; 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)-butoxy]-3,4-dihydro-1H-quinolin-2-one; 89332-50-3
Click to Show/Hide
|
||||
| Species Origin | Broussonetia ... | Click to Show/Hide | |||
| Broussonetia | |||||
| Disease | Atherosclerosis [ICD-11: BD40] | Approved | [1] | ||
| Structure |
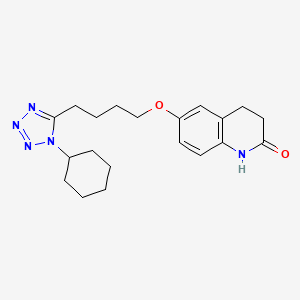
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-4.853
MDCK Permeability
-4.745
PAMPA
- - -
HIA
- - -
Distribution
VDss
-0.125
PPB
96.2%
BBB
- - -
Metabolism
CYP1A2 inhibitor
- - -
CYP1A2 substrate
- -
CYP2C19 inhibitor
- - -
CYP2C19 substrate
+++
CYP2C9 inhibitor
- - -
CYP2C9 substrate
- - -
CYP2D6 inhibitor
- - -
CYP2D6 substrate
- - -
CYP3A4 inhibitor
+++
CYP3A4 substrate
+++
CYP2B6 inhibitor
- - -
CYP2B6 substrate
- - -
CYP2C8 inhibitor
- -
HLM Stability
+++
Excretion
CLplasma
7.243
T1/2
0.232
Toxicity
DILI
+
Rat Oral Acute Toxicity
+
FDAMDD
++
Respiratory
++
Human Hepatotoxicity
++
Ototoxicity
++
Drug-induced Nephrotoxicity
+
Drug-induced Neurotoxicity
+
Hematotoxicity
+
Genotoxicity
++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C20H27N5O2
|
||||
| PubChem CID | |||||
| Canonical SMILES |
C1CCC(CC1)N2C(=NN=N2)CCCCOC3=CC4=C(C=C3)NC(=O)CC4
|
||||
| InChI |
1S/C20H27N5O2/c26-20-12-9-15-14-17(10-11-18(15)21-20)27-13-5-4-8-19-22-23-24-25(19)16-6-2-1-3-7-16/h10-11,14,16H,1-9,12-13H2,(H,21,26)
|
||||
| InChIKey |
RRGUKTPIGVIEKM-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 73963-72-1
|
||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Pravastatin | Hyper-lipoproteinaemia | Click to Show/Hide the Molecular Data of This Drug | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Molecule(s)
Regulation |
Down-regulation | Expression | ICAM1 | Molecule Info |
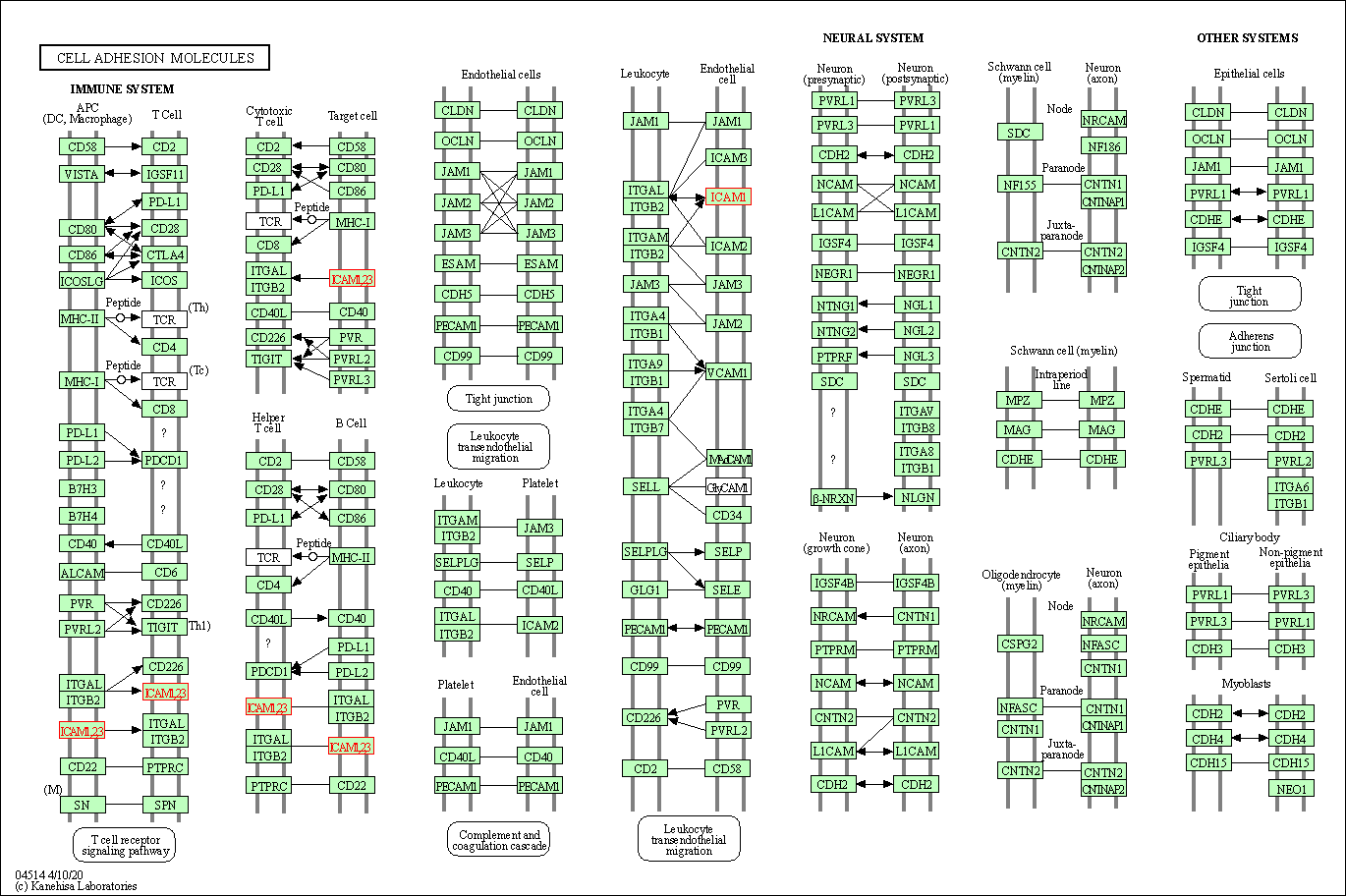
Pathway MAP
|
|
| In-vivo Model | For a xenograft model, Ten-week-old LDLR KO mice were fed a high-fat, high cholesterol diet. | |||||
| Experimental
Result(s) |
Combination therapy with pravastatin and cilostazol exerts beneficial effects by decreasing atherosclerotic lesion progression and improving the proinflammatory state in the vascular endothelium. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Phosphodiesterase 3A (PDE3A) | Molecule Info | [3] | |
| KEGG Pathway | Purine metabolism | Click to Show/Hide | ||
| 2 | cGMP-PKG signaling pathway | |||
| 3 | cAMP signaling pathway | |||
| 4 | Morphine addiction | |||
| Reactome | cGMP effects | Click to Show/Hide | ||
| 2 | G alpha (s) signalling events | |||
| WikiPathways | miR-targeted genes in muscle cell - TarBase | Click to Show/Hide | ||
| 2 | miR-targeted genes in lymphocytes - TarBase | |||