Natural Product (NP) Details
| General Information of the NP (ID: NP9583) | |||||
|---|---|---|---|---|---|
| Name |
Paroxetine
|
||||
| Synonyms |
paroxetine; 61869-08-7; Paxil; Paxil CR; Casbol; Seroxat; Paroxetina; Paroxetinum; Aropax; Paroxetinum [INN-Latin]; Paroxetina [INN-Spanish]; BRL 29060; Paxetil; FG 7051; Pexeva; Frosinor; Motivan; PaxPar; (3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine; (3s,4r)-3-((benzo[d][1,3]dioxol-5-yloxy)methyl)-4-(4-fluorophenyl)piperidine; BRL-29060; UNII-41VRH5220H; (-)-(3S,4R)-4-(p-Fluorophenyl)-3-((3,4-(methylenedioxy)phenoxy)methyl)piperidine; [3H]Paroxetine; FG-7051; CHEMBL490; (3S,4R)-3-(1,3-benzodioxol-5-yloxymethyl)-4-(4-fluorophenyl)piperidine; Piperidine, 3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)-, (3S,4R)-; CHEBI:7936; 41VRH5220H; C19H20FNO3; 110429-35-1; DSSTox_CID_3425; (3S,4R)-3-[(2H-1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine; (3S-trans)-3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)piperidine; Paroxetine (TN); DSSTox_RID_77022; Piperidine, 3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)-, (3S-trans)-; DSSTox_GSID_23425; Paroxetine [USAN:INN:BAN]; CHEMBL1708; Paroxetine Base; piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-, (3S,4R)-; Piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-,(3S,4R)-; (-)-Paroxetine; PAROXETINE HCL HEMIHYDRATE; CAS-61869-08-7; Paroxetine (USP/INN); 3h-paroxetine; Paroxetine.HCl; Piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-, (3S-trans)-; NCGC00182968-01; 8PR; NNC-20-7051; Spectrum_001752; SpecPlus_000788; Prestwick3_000851; Spectrum5_001665; (-)-(3S,4R)-4-(p-Fluorophenyl)-3-((3,4-methylenedioxy)phenoxy)methyl)piperidine; SCHEMBL27799; BSPBio_000861; KBioSS_002232; BIDD:GT0673; DivK1c_006884; BPBio1_000949; GTPL4790; DTXSID3023425; BDBM22416; HSDB 7175; KBio1_001828; KBio2_002232; KBio2_004800; KBio2_007368; HMS2090H05; ZINC527386; Tox21_113123; BDBM50331515; (-)-trans-4-(4-Fluorophenyl)-3-(3,4-methylenedioxyphenoxymethyl)piperidine; AKOS015888636; Tox21_113123_1; AC-8185; DB00715; SDCCGSBI-0051908.P003; (-)-trans-4-(p-fluorophenyl)-3-[[3,4-(methylenedioxy)phenoxy]methyl]-piperidine; (3S-trans)-3-[(1,3-Benzodioxol-5-yl-oxy)methyl]-4-(4-fluorophenyl)piperidine; NCGC00025355-02; NCGC00025355-03; NCGC00025355-04; NCGC00025355-05; NCGC00025355-06; NCGC00025355-07; NCGC00025355-08; NCGC00025355-09; NCGC00025355-12; NCGC00025355-22; SBI-0051908.P002; AB00514724; C07415; D02362; AB00053704-21; AB00053704_22; AB00053704_23; Paroxetine Hydrochloride Anhydrous EP Impurity E; 869P087; paroxetine hydrochloride (anhydrous or hemihydrate); Q408471; BRD-K37991163-003-02-7; BRD-K37991163-050-05-1; cis-Paroxetine; Paroxetine USP Related Compound D;[(3S,4R)-4-(p-Fluorophenyl)-3-piperidyl](1,3-dioxa-5-indanyloxy)methane
Click to Show/Hide
|
||||
| Species Origin | Areca catechu ... | Click to Show/Hide | |||
| Areca catechu | |||||
| Disease | Depression [ICD-11: 6A70-6A71] | Approved | [1] | ||
| Structure |
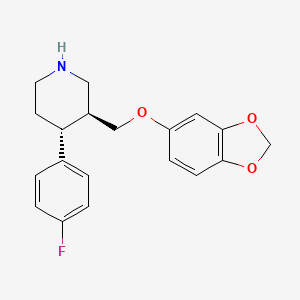
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-5.178
MDCK Permeability
-4.929
PAMPA
+
HIA
- - -
Distribution
VDss
1.178
PPB
88.7%
BBB
+++
Metabolism
CYP1A2 inhibitor
+++
CYP1A2 substrate
+++
CYP2C19 inhibitor
- - -
CYP2C19 substrate
+++
CYP2C9 inhibitor
- - -
CYP2C9 substrate
- - -
CYP2D6 inhibitor
- -
CYP2D6 substrate
+++
CYP3A4 inhibitor
+++
CYP3A4 substrate
++
CYP2B6 inhibitor
+++
CYP2B6 substrate
- - -
CYP2C8 inhibitor
- - -
HLM Stability
- - -
Excretion
CLplasma
5.045
T1/2
0.603
Toxicity
DILI
++
Rat Oral Acute Toxicity
++
FDAMDD
+
Respiratory
+++
Human Hepatotoxicity
++
Ototoxicity
+
Drug-induced Nephrotoxicity
+++
Drug-induced Neurotoxicity
+++
Hematotoxicity
+
Genotoxicity
++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C19H20FNO3
|
||||
| PubChem CID | |||||
| Canonical SMILES |
C1CNCC(C1C2=CC=C(C=C2)F)COC3=CC4=C(C=C3)OCO4
|
||||
| InChI |
1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1
|
||||
| InChIKey |
AHOUBRCZNHFOSL-YOEHRIQHSA-N
|
||||
| CAS Number |
CAS 61869-08-7
|
||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Everolimus | Renal cell carcinoma | Click to Show/Hide the Molecular Data of This Drug | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vitro Model | SH-SY5Y | CVCL_0019 | Neuroblastoma | Homo sapiens | ||
| Experimental
Result(s) |
Combination treatment with Everolimus and Paroxetine showed synergistic post-ischemic neuroprotective efficacy. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Serotonin transporter (SERT) | Molecule Info | [3] | |
| KEGG Pathway | Serotonergic synapse | Click to Show/Hide | ||
| NetPath Pathway | TCR Signaling Pathway | Click to Show/Hide | ||
| Panther Pathway | 5HT1 type receptor mediated signaling pathway | Click to Show/Hide | ||
| 2 | 5HT2 type receptor mediated signaling pathway | |||
| 3 | 5HT3 type receptor mediated signaling pathway | |||
| 4 | 5HT4 type receptor mediated signaling pathway | |||
| WikiPathways | Monoamine Transport | Click to Show/Hide | ||
| 2 | SIDS Susceptibility Pathways | |||
| 3 | NRF2 pathway | |||
| 4 | Synaptic Vesicle Pathway | |||
| 5 | Serotonin Transporter Activity | |||

