Natural Product (NP) Details
| General Information of the NP (ID: NP9823) | |||||
|---|---|---|---|---|---|
| Name |
Xanthotoxin
|
||||
| Synonyms |
methoxsalen; 8-Methoxypsoralen; 298-81-7; XANTHOTOXIN; Ammoidin; Meladinine; Xanthotoxine; 8-MOP; Meloxine; Oxsoralen; 9-Methoxy-7H-furo[3,2-g]chromen-7-one; Meladinin; Oxypsoralen; Puvalen; Meladoxen; Ammodin; Uvadex; Methoxa-Dome; 8-Methoxypsoralene; Oxsoralen-ultra; New-Meladinin; Geroxalen; Methoxalen; Puvamet; Oxsoralen lotion; Zanthotoxin; Psoralon-MOP; 9-Methoxypsoralen; 8-MP; 8-Methoxyfuranocoumarin; 9-methoxyfuro[3,2-g]chromen-7-one; 9-Methoxy-7H-furo[3,2-g][1]benzopyran-7-one; O-methylxanthotoxol; 8-Methoxy-2',3',6,7-furocoumarin; 7H-Furo[3,2-g][1]benzopyran-7-one, 9-methoxy-; NCI-C55903; Methoxy-8-psoralen; 8-Methoxy-4',5',6,7-furocoumarin; 6-Hydroxy-7-methoxy-5-benzofuranacrylic acid delta-lactone; Methoxsalen-d3; UNII-U4VJ29L7BQ; 8-Methoxy-[furano-3'.2':6.7-coumarin]; NSC45923; MFCD00005009; NSC 45923; 8-Methoxy-4',5':6,7-furocoumarin; 8-Methoxy-(furano-3'.2':6.7-coumarin); 9-Methoxyfuro[3,2-g][1]benzopyran-7-one; Methoxsalen (Oxsoralen); 9-Methoxy-7H-furo(3,2-g)(1)benzopyran-7-one; 9-Methoxyfuro(3,2-g)chromen-7-one; CHEMBL416; Oxsoralen Ultra; 7H-Furo(3,2-G)(1)benzopyran-7-one, 9-methoxy-; U4VJ29L7BQ; 8-Methoxy-6,7-furanocoumarin; CHEBI:18358; 9-(methyloxy)-7H-furo[3,2-g]chromen-7-one; Proralone-mop; NSC-45923; 9-methoxyfurano[3,2-g]chromen-2-one; Meladinin (VAN); NCGC00016418-07; CAS-298-81-7; Methoxaten; Oxoralen; ST041029; DSSTox_CID_830; 8-Methoxypsoralen, 99%; Ultra Mop; DSSTox_RID_75816; DSSTox_GSID_20830; 1246819-63-5; Deltapsoralen; Dltasoralen; Methoxsalene; Metoxaleno; Methoxsalen, 8-; SMR000071170; CCRIS 2083; HSDB 2505; 8-Methoxypsoralen with ultraviolet A therapy; OXSORALEN (TN); SR-01000629727; EINECS 206-066-9; Methoxsalen plus ultraviolet radiation; BRN 0196453; Vitpso; Methoxsalen [USP:BAN:JAN]; 9-Methoxy-7H-furo(3,2-g)benzopyran-7-one; 5-Benzofuranacrylic acid, 6-hydroxy-7-methoxy-, delta-lactone; Prestwick_661; Spectrum_001023; 5-Demethoxyisoimpinellin; 8-Methoxy(furano-3'.2':6.7-coumarin); Prestwick0_000479; Prestwick1_000479; Prestwick2_000479; Prestwick3_000479; Spectrum2_001052; Spectrum3_000499; Spectrum4_000052; Spectrum5_001891; UVADEX (TN); Methoxsalen (JP17/USP); 7H-Furo[3, 9-methoxy-; Oprea1_166319; SCHEMBL19168; BSPBio_000618; BSPBio_001997; KBioGR_000543; KBioSS_001503; 5-19-06-00015 (Beilstein Handbook Reference); 8MO; MLS000062633; MLS002303011; DivK1c_000763; SPECTRUM1500400; SPBio_001004; SPBio_002557; BPBio1_000680; MEGxp0_000095; Xanthotoxin, analytical standard; DTXSID8020830; ACon1_000174; HMS502G05; KBio1_000763; KBio2_001503; KBio2_004071; KBio2_006639; KBio3_001497; 8-Methoxy Psoralen-[13C,d3]; 8-Methoxy-2',6,7-furocoumarin; 8-Methoxy-4',6,7-furocoumarin; NINDS_000763; HMS1569O20; HMS1920N05; HMS2091D20; HMS2096O20; HMS2269P03; HMS3259L13; HMS3655B05; HMS3884K16; Pharmakon1600-01500400; 5-Benzofuranacrylic acid, 6-hydroxy-7-methoxy-, .delta.-lactone; BCP28212; SM-88 COMPONENT METHOXSALEN; ZINC2548959; Tox21_110432; Tox21_201767; Tox21_302816; 8-Methoxypsoralen;Xanthotoxin;8-MOP; BDBM50041234; CCG-36366; NSC757114; s1952; SBB005950; STK735539; WLN: T C566 DO LVOJ BO1; 8-Methoxypsoralen, analytical standard; AKOS000277012; Tox21_110432_1; 9-Methoxy-furo[3,2-g]chromen-7-one; AC-4259; AM84906; CS-1983; DB00553; DS-5159; MCULE-2500932325; NC00652; NSC-757114; SDCCGMLS-0042377.P002; IDI1_000763; 8-methoxy-2'',3'',6,7-furocoumarin; 8-methoxy-4'',5'':6,7-furocoumarin; NCGC00016418-01; NCGC00016418-02; NCGC00016418-03; NCGC00016418-04; NCGC00016418-05; NCGC00016418-06; NCGC00016418-08; NCGC00016418-09; NCGC00016418-10; NCGC00016418-11; NCGC00016418-12; NCGC00016418-14; NCGC00016418-15; NCGC00060938-02; NCGC00060938-03; NCGC00060938-04; NCGC00060938-05; NCGC00060938-06; NCGC00178871-01; NCGC00178871-02; NCGC00178871-03; NCGC00256435-01; NCGC00259316-01; AK111265; HY-30151; NCI60_004085; Q039; SBI-0051443.P003; FT-0602101; FT-0671145; N1305; SW167762-4; 8-methoxy-[furano-3''.2'':6.7-coumarin]; 9-Methoxy-7H-furo[3,2-g]chromen-7-one #; EN300-52504; C01864; D00139; J10204; 9-(Trideuteriomethoxy)furo[3,2-g]chromen-7-one; AB00052042-14; AB00052042-15; AB00052042_16; AB00052042_17; 298M817; 9-METHOXY-2H-FURO[3,2-G]CHROMEN-2-ONE; 9-Methoxy-7H-furo[3,2- g][1]benzopyran-7-one; Q408570; Q-100381; SR-01000629727-2; SR-01000629727-4; BRD-K63430059-001-03-2; BRD-K63430059-001-06-5; BRD-K63430059-001-09-9; Z1258578369; Methoxsalen, United States Pharmacopeia (USP) Reference Standard; 12692-94-3
Click to Show/Hide
|
||||
| Species Origin | Cullen corylifolium ... | Click to Show/Hide | |||
| Cullen corylifolium | |||||
| Disease | Mycosis fungoides [ICD-11: 2B01] | Approved | [1] | ||
| Structure |
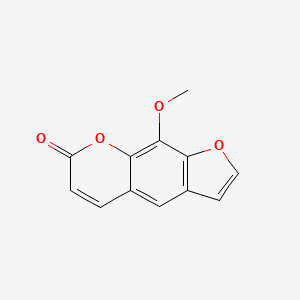
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-4.348
MDCK Permeability
-4.562
PAMPA
+
HIA
- - -
Distribution
VDss
-0.236
PPB
94.6%
BBB
- - -
Metabolism
CYP1A2 inhibitor
+++
CYP1A2 substrate
- -
CYP2C19 inhibitor
+++
CYP2C19 substrate
- - -
CYP2C9 inhibitor
- -
CYP2C9 substrate
- - -
CYP2D6 inhibitor
+++
CYP2D6 substrate
- - -
CYP3A4 inhibitor
+++
CYP3A4 substrate
+++
CYP2B6 inhibitor
+++
CYP2B6 substrate
- - -
CYP2C8 inhibitor
+++
HLM Stability
- - -
Excretion
CLplasma
12.283
T1/2
1.114
Toxicity
DILI
++
Rat Oral Acute Toxicity
+
FDAMDD
++
Respiratory
++
Human Hepatotoxicity
-
Ototoxicity
- -
Drug-induced Nephrotoxicity
- -
Drug-induced Neurotoxicity
-
Hematotoxicity
- -
Genotoxicity
+++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C12H8O4
|
||||
| PubChem CID | |||||
| Canonical SMILES |
COC1=C2C(=CC3=C1OC=C3)C=CC(=O)O2
|
||||
| InChI |
1S/C12H8O4/c1-14-12-10-8(4-5-15-10)6-7-2-3-9(13)16-11(7)12/h2-6H,1H3
|
||||
| InChIKey |
QXKHYNVANLEOEG-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 298-81-7
|
||||
| ChEBI ID | |||||
| Herb ID | |||||
| SymMap ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Levetiracetam + Valproic acid | Click to Show/Hide the Molecular Data of This Drug | |||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Adult male Albino Swiss mice weighing 22-26 g were used in this study. | |||||
| Experimental
Result(s) |
XANT in combination with levetiracetam exerts beneficial anticonvulsant pharmacodynamic interactions in the 6 Hz mouse psychomotor seizure model. | |||||
| Ketoconazole + Amphotericin B | Click to Show/Hide the Molecular Data of This Drug | |||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [3] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vitro Model | Candida albicans ATCC 22972 | Microorganism model | Candida albicans | |||
| Candida glabrata ATCC 90525 | Microorganism model | Candida glabrata | ||||
| Candida guilliermondii ATCC 20216 | Microorganism model | Candida guilliermondii | ||||
| Candida krusei ATCC 6258 | Microorganism model | Candida krusei | ||||
| Candida parapsilosis ATCC 7330 | Microorganism model | Candida parapsilosis | ||||
| Candida tropicalis ATCC 42678 | Microorganism model | Candida tropicalis | ||||
| Experimental
Result(s) |
1/2 MIC dose of xanthorrhizol in combination with 1/2 MIC dose of ketoconazole or 1/2 MIC dose of amphotericin B exhibited growth inhibition of all Candida species tested and reduced viable cells by several logs within 4 h. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Cytochrome P450 2A6 (CYP2A6) | Molecule Info | [4] | |

