Drug Combination Details
| General Information of the Combination (ID: C60150) | |||||
|---|---|---|---|---|---|
| Name | Misoprostol NP Info | + | Mifepristone Drug Info | ||
| Structure |
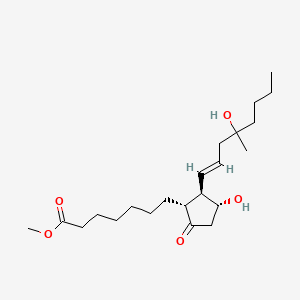
|
+ |

|
||
| Disease |
Abortion
[ICD-11: JA00]
|
Phase 4 | [1] | ||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. Enhancing Drug Efficacy by This Combination | ||||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Experiment 1 Reporting the Effect of This Combination | [2] | |||||
| In-vivo Model | Retrospective clinical study | |||||
| Experimental
Result(s) |
The addition of mifepristone to medical treatment regimens for first trimester miscarriage significantly decreased the need for repeat medical dosing and surgical curettage. | |||||

