Drug Details
| General Information of the Drug (ID: DR1480) | ||||
|---|---|---|---|---|
| Name |
Parecoxib
|
|||
| Synonyms |
Parecoxib; 198470-84-7; Dynastat; SC-69124; SC 69124; CHEBI:73038; UNII-9TUW81Y3CE; N-((p-(5-methyl-3-phenyl-4-isoxazolyl)phenyl)sulfonyl)propionamide; 9TUW81Y3CE; N-[4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)phenyl]sulfonylpropanamide; N-[4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzenesulfonyl]propanamide; Propanamide, N-[[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]-; Valus-P; Vorth-P; Parocoxib; Propanamide, N-((4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl)sulfonyl)-; N-{[4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)phenyl]sulfonyl}propanamide; parecoxibum; Parecoxib [USAN:INN:BAN]; Valdecoxib Impurity J; Parecoxib (USAN/INN); SCHEMBL9529; GTPL2895; CHEMBL1206690; DTXSID1044229; HMS3741C09; HMS3885L14; AMY42103; BCP07254; EX-A1993; ZINC5761797; BDBM50506744; CP0130; s4656; AKOS025401810; CCG-268299; CS-1959; DB08439; SC69124; NCGC00181773-03; NCGC00181773-05; NCGC00378576-02; Parecoxib 100 microg/mL in Acetonitrile; AC-25842; AS-72353; HY-17474; FT-0697932; Y1120; Parecoxib, VETRANAL(TM), analytical standard; D03716; Q347941; J-523329; BRD-K13800121-236-01-7; N-[4-(5-Methyl-3-phenyl-oxazol-4-yl)phenyl]sulfonylpropanamde; n-[[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]-propanamide; n-[[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]propanamide; N-{[4-(5-methyl-3-phenylisoxazol-4-yl)phenyl]sulfonyl}propanamide; N-[[4-(5-methyl-3-phenyl-4-isoxazolyl) phenyl] sulfonyl] propanamide; N-?[[4-?(5-?Methyl-?3-?phenyl-?4-?isoxazolyl)?phenyl]?sulfonyl]?propanamide; PXB
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Gastrointestinal stromal tumor [ICD-11: 2B5B] | Phase 4 | [1] | |
| Structure |
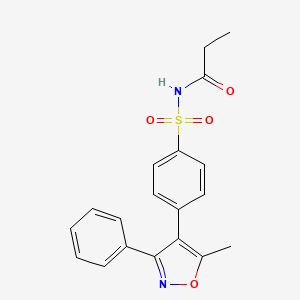
|
Click to Download Mol2D MOL |
||
| ADMET Property |
Half-life
The concentration or amount of drug in body reduced by one-half in 22 minutes (parecoxib); 8 hours (valdecoxib)
Metabolism
The drug is metabolized via the hepatic
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C19H18N2O4S
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CCC(=O)NS(=O)(=O)C1=CC=C(C=C1)C2=C(ON=C2C3=CC=CC=C3)C
|
|||
| InChI |
1S/C19H18N2O4S/c1-3-17(22)21-26(23,24)16-11-9-14(10-12-16)18-13(2)25-20-19(18)15-7-5-4-6-8-15/h4-12H,3H2,1-2H3,(H,21,22)
|
|||
| InChIKey |
TZRHLKRLEZJVIJ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 198470-84-7
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Phloroglucinol | Dryopteris arguta | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Experimental
Result(s) |
Parecoxib in combination with phloroglucinol for acute renal colic has a faster action, also reduces the demand of rescue analgesics. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Lactotransferrin (LTF) | Molecule Info | [3] | |
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | Click to Show/Hide | ||
| 2 | TCR Signaling Pathway | |||
| Reactome | ROS production in response to bacteria | Click to Show/Hide | ||
| 2 | Amyloid formation | |||
| WikiPathways | Latent infection of Homo sapiens with Mycobacterium tuberculosis | Click to Show/Hide | ||

