Drug Details
| General Information of the Drug (ID: DR2114) | ||||
|---|---|---|---|---|
| Name |
Lamotrigine
|
|||
| Synonyms |
lamotrigine; 84057-84-1; 6-(2,3-Dichlorophenyl)-1,2,4-triazine-3,5-diamine; Lamictal; Lamictal Cd; Lamictal XR; Lamotriginum [Latin]; Lamotrigina [Spanish]; Lamotriginum; BW 430C; BW-430C; Lamotrigina; Lamictal ODT; 3,5-Diamino-6-(2,3-dichlorophenyl)-1,2,4-triazine; 1,2,4-Triazine-3,5-diamine, 6-(2,3-dichlorophenyl)-; 3,5-Diamino-6-(2,3-dichlorophenyl)-as-triazine; C9H7Cl2N5; LTG;BW430C; UNII-U3H27498KS; MFCD00865333; CHEMBL741; MLS000069685; CHEBI:6367; U3H27498KS; Lamotrigine, 98%; Lamotrigine-13C3; NCGC00015605-06; Labileno; Lamitor; SMR000058464; DSSTox_CID_3195; DSSTox_RID_76918; DSSTox_GSID_23195; Lamictal (TN); CAS-84057-84-1; BW430C; SR-01000000187; EINECS 281-901-8; HSDB 7526; EUR-1048; Lamotrigine [USAN:USP:INN:BAN]; zine-3,5-diamine; GI 267119X; 1188265-38-4; Lamotrigine-13C-d3; Opera_ID_12; Tocris-1611; hydroxymethyl progesterone; Lopac-L-3791; L 3791; Lopac0_000688; SCHEMBL35439; MLS000759486; MLS001077325; MLS001423991; BIDD:GT0794; Lamotrigine (JAN/USP/INN); Lamotrigine, >=98%, powder; GTPL2622; TRI020; DTXSID2023195; ZINC13156; HMS2051C10; HMS2089M08; HMS2093P21; HMS2230L04; HMS3262I17; HMS3268G17; HMS3371O16; HMS3393C10; HMS3657A17; HMS3715H21; HMS3885M03; Pharmakon1600-01505610; AMY40805; BCP12156; HY-B0495; Lamotrigine 1.0 mg/ml in Methanol; Tox21_110179; Tox21_500688; ANW-44921; BDBM50031299; NSC746307; NSC759171; s3024; STK628377; AKOS005561147; Tox21_110179_1; 6-(2,2,4-triazine-3,5-diyldiamine; CCG-100856; DB00555; KS-1074; LP00688; MCULE-7648410888; NC00106; NE61394; NSC 746307; NSC 759171; NSC-746307; NSC-759171; SDCCGSBI-0050666.P003; SMP2_000303; NCGC00015605-01; NCGC00015605-02; NCGC00015605-03; NCGC00015605-04; NCGC00015605-05; NCGC00015605-07; NCGC00015605-08; NCGC00015605-09; NCGC00015605-10; NCGC00015605-23; NCGC00015605-24; NCGC00022936-02; NCGC00022936-04; NCGC00022936-05; NCGC00261373-01; AC-10298; AC-32483; AK-72807; AT-15488; K499; Lamotrigine 100 microg/mL in Acetonitrile; SBI-0050666.P002; 6-(2,3-Dichloro-phenyl)-[1,2,4]tria; AB0014255; DB-014839; B2249; EU-0100688; FT-0602546; FT-0670713; FT-0670714; L-205; L0241; SW197486-3; 57L841; A11873; D00354; J10032; AB00384359-16; AB00384359_17; AB00384359_18; A840709; Q410346; 3,5-diamino-(2,3-dichlorophenyl)-1,2,4-triazine; Q-201221; SR-01000000187-2; SR-01000000187-4; SR-01000000187-7; BRD-K93460210-071-01-6; SR-01000000187-10; 3,5-diamino-6-(2,3,-dichlorophenyl)-1,2,4-triazine; 6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine.; F2173-0540; Z1550648755; 6-(2,3-Dichloro-phenyl)-[1,2,4]triazine-3,5-diamine; 6-[2,3-bis(chloranyl)phenyl]-1,2,4-triazine-3,5-diamine; Lamotrigine, British Pharmacopoeia (BP) Reference Standard; Lamotrigine, European Pharmacopoeia (EP) Reference Standard; 6-(2,3-Dichloro-phenyl)-[1,2,4]triazine-3,5-diamine(lamotrigine); GI 267119X; 6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine; Lamotrigine, United States Pharmacopeia (USP) Reference Standard; Lamotrigine, Pharmaceutical Secondary Standard; Certified Reference Material; Lamotrigine for peak identification, European Pharmacopoeia (EP) Reference Standard; Lamotrigine for system suitability, European Pharmacopoeia (EP) Reference Standard; Lamotrigine solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Bipolar disorder [ICD-11: 6A60] | Approved | [1] | |
| Structure |
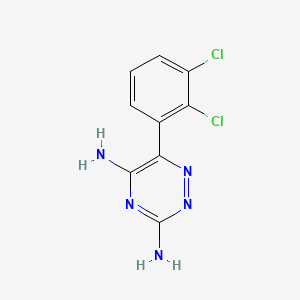
|
Click to Download Mol2D MOL |
||
| ADMET Property |
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 2: low solubility and high permeability
Bioavailability
97.6% of drug becomes completely available to its intended biological destination(s)
Clearance
The clearance of drug is 0.18-1.21 mL/min/kg
Elimination
Following oral administration of 240 mg radiolabelled lamotrigine, about 94% of total drug and its metabolites administered is recovered in the urine and 2% is recovered in the feces
Half-life
The concentration or amount of drug in body reduced by one-half in 14 - 59 hours
Metabolism
The drug is metabolized via the glucuronidated, forming 2-N-glucuronide conjugate
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 27.89137 micromolar/kg/day
Vd
The volume of distribution (Vd) of drug is 0.9-1.3 L/kg
Water Solubility
The ability of drug to dissolve in water is measured as 0.17 mg/mL
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C9H7Cl2N5
|
|||
| PubChem CID | ||||
| Canonical SMILES |
C1=CC(=C(C(=C1)Cl)Cl)C2=C(N=C(N=N2)N)N
|
|||
| InChI |
1S/C9H7Cl2N5/c10-5-3-1-2-4(6(5)11)7-8(12)14-9(13)16-15-7/h1-3H,(H4,12,13,14,16)
|
|||
| InChIKey |
PYZRQGJRPPTADH-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 84057-84-1
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Sodium channel alpha Nav1.9 (SCN11A) | Molecule Info | [2] | |
| Reactome | Interaction between L1 and Ankyrins | Click to Show/Hide | ||
