Drug Details
| General Information of the Drug (ID: DR2744) | ||||
|---|---|---|---|---|
| Name |
Deprenyl
|
|||
| Synonyms |
Deprenyl; Deprenil; 2323-36-6; PHENETHYLAMINE, N,alpha-DIMETHYL-N-2-PROPYNYL-; CHEBI:50217; methyl(1-phenylpropan-2-yl)(prop-2-yn-1-yl)amine; (+-)-Deprenyl; Selegilinum [INN-Latin]; Selegilina [INN-Spanish]; dl-Deprenyl; UNII-DPF682Q08V; Selegiline D5; DPF682Q08V; N-methyl-1-phenyl-N-prop-2-ynylpropan-2-amine;hydrochloride; (+/-)-Deprenyl; (.+/-.)-Deprenyl; N,alpha-Dimethyl-N-2-propynylphenethylamine; 1,N-Dimethyl-N-propargyl-2-phenylethylamine; N,alpha-Dimethyl-N-2-propynylbenzeneethanamine; CHEMBL8663; SCHEMBL74753; cid_92913; BDBM39862; DTXSID60860142; HMS2089B08; MCULE-3188554308; NCGC00015624-03; NCGC00016708-02; NCGC00162273-01; SBI-0206908.P001; FT-0665904; N,.alpha.-Dimethyl-N-2-propynylphenethylamine; AB00489975_10; DEP_188.1433_10.1; Phenethylamine, N,.alpha.-dimethyl-N-2-propynyl-; Q402633; Benzeneethanamine, N,.alpha.-dimethyl-N-2-propynyl-; BRD-A28545468-003-10-9; N-methyl-N-(1-methyl-2-phenylethyl)prop-2-yn-1-amine; Benzeneethanamine, N,alpha-dimethyl-N-2-propynyl- (9CI); N-Methyl-N-(1-methyl-2-phenylethyl)-2-propyn-1-amine #; methyl-(1-methyl-2-phenyl-ethyl)-propargyl-amine;hydrochloride; N-methyl-1-phenyl-N-prop-2-ynyl-2-propanamine;hydrochloride; N-methyl-1-phenyl-N-prop-2-ynyl-propan-2-amine;hydrochloride; METHYL-(1-METHYL-2-PHENYL-ETHYL)-PROP-2-YNYL-AMINE HYDROCHLORIDE
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Parkinson's disease [ICD-11: 8A00] | Phase 4 | [1] | |
| Structure |
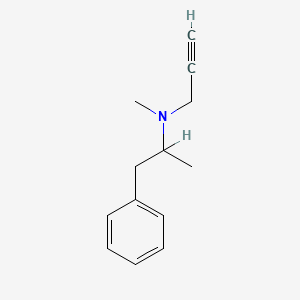
|
Click to Download Mol2D MOL |
||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C13H17N
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CC(CC1=CC=CC=C1)N(C)CC#C
|
|||
| InChI |
1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3
|
|||
| InChIKey |
MEZLKOACVSPNER-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 2323-36-6
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Physostigmine | Physostigma venenosum | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Experimental
Result(s) |
Based on earlier positive findings in Alzheimer patients with the monoamine oxidase B inhibitor, 1-deprenyl, the authors speculate that a combination of physostigmine, the short-acting cholinesterase inhibitor, and 1-deprenyl might be more beneficial than either agent alone. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Monoamine oxidase B (MAO-B) | Molecule Info | [3] | |
| BioCyc | Superpathway of tryptophan utilization | Click to Show/Hide | ||
| 2 | Tryptophan degradation via tryptamine | |||
| 3 | Dopamine degradation | |||
| 4 | Putrescine degradation III | |||
| 5 | Noradrenaline and adrenaline degradation | |||
| KEGG Pathway | Glycine, serine and threonine metabolism | Click to Show/Hide | ||
| 2 | Arginine and proline metabolism | |||
| 3 | Histidine metabolism | |||
| 4 | Tyrosine metabolism | |||
| 5 | Phenylalanine metabolism | |||
| 6 | Tryptophan metabolism | |||
| 7 | Drug metabolism - cytochrome P450 | |||
| 8 | Metabolic pathways | |||
| 9 | Serotonergic synapse | |||
| 10 | Dopaminergic synapse | |||
| 11 | Cocaine addiction | |||
| 12 | Amphetamine addiction | |||
| 13 | Alcoholism | |||
| Panther Pathway | Adrenaline and noradrenaline biosynthesis | Click to Show/Hide | ||
| 2 | 5-Hydroxytryptamine degredation | |||
| 3 | Dopamine receptor mediated signaling pathway | |||
| Pathway Interaction Database | Alpha-synuclein signaling | Click to Show/Hide | ||
| WikiPathways | Tryptophan metabolism | Click to Show/Hide | ||
| 2 | Dopamine metabolism | |||
| 3 | Phase 1 - Functionalization of compounds | |||

