Drug Details
| General Information of the Drug (ID: DR3098) | ||||
|---|---|---|---|---|
| Name |
6-mercaptopurine
|
|||
| Synonyms |
6-Mercaptopurine; mercaptopurine; 50-44-2; Purinethol; Mercapurin; 6-Thiopurine; Leukerin; Leupurin; Mercaleukin; 6-Thioxopurine; Puri-Nethol; Ismipur; 7H-purine-6-thiol; Mern; 6-Thiohypoxanthine; 6-Mercaptopurin; 6-Purinethiol; Purimethol; Purinethiol; 6-MP; 1,9-dihydro-6H-purine-6-thione; Purine-6-thiol; 3H-Purine-6-thiol; 6 MP; Mercaleukim; 9H-Purine-6-thiol; 1,7-Dihydro-6H-purine-6-thione; Hypoxanthine, thio-; Mercaptopurine (6-MP); 3,7-dihydropurine-6-thione; 6H-Purine-6-thione, 1,7-dihydro-; Mercaptopurin; Mercaptopurina; Mercaptopurinum; Merkaptopuryna; Xaluprine; 6-Merkaptopurin; Purine, 6-mercapto-; 9H-Purine-6(1H)-thione; Mercaptopurine anhydrous; Purine-6(1H)-thione; NCI-C04886; 7-Mercapto-1,3,4,6-tetrazaindene; 1H-Purine, 6-mercapto-; NSC 755; U-4748; NSC-755; UNII-PKK6MUZ20G; Mercaptopurine;6-MP; NSC755; 3,7-dihydro-6H-purine-6-thione; Purinethol (TN); PKK6MUZ20G; 1,9-dihydropurine-6-thione; 1H-purine-6(7H)-thione.; CHEBI:2208; 157930-13-7; Mercaptopurine (VAN); DSSTox_CID_810; Mercaptopurin [German]; Merkaptopuryna [Polish]; 6-Merkaptopurin [Czech]; DSSTox_RID_75801; DSSTox_GSID_20810; Mercaptopurine (anhydrous); 157930-14-8; Mercaptopurinum [INN-Latin]; Mercaptopurina [INN-Spanish]; Purixan; thiohypoxanthine; Purine-6-thiol, monohydrate; CAS-50-44-2; SMR000544948; Mercaptopurine, 6-; CCRIS 2761; Mercaptopurine (INN); HSDB 3235; SR-05000001925; 1194-62-3; EINECS 200-037-4; NCIMech_000025; 9H-Purin-6-yl hydrosulfide; a thiopurine; Mercaptopurine;; Mercaptopurine [USAN:USP:INN]; PubChem9680; Spectrum_000921; ACMC-1AUNA; Spectrum2_000060; Spectrum3_000491; Spectrum4_000857; Spectrum5_000950; M0063; H-Purine-6(1H)-thione; Azathioprine EP Impurity B; SCHEMBL3893; CHEMBL1425; BSPBio_001981; KBioGR_001493; KBioGR_002363; KBioSS_001401; KBioSS_002366; AG-670/31547064; MLS001066623; MLS001304020; MLS001304953; MLS006011869; ARONIS27054; DivK1c_000493; SPECTRUM1500387; SPBio_000219; GTPL7226; SCHEMBL2790086; 7H-Purin-6-yl hydrosulfide #; DTXSID0020810; SCHEMBL12683725; CHEBI:50667; CHEBI:94796; HMS501I15; KBio1_000493; KBio2_001401; KBio2_002363; KBio2_003969; KBio2_004931; KBio2_006537; KBio2_007499; KBio3_001481; KBio3_002842; 7-Mercapto-1,4,6-tetrazaindene; cMAP_000033; NINDS_000493; 6,7-dihydro-3H-purine-6-thione; HMS1920L07; HMS2091B20; HMS2236L06; HMS3259N03; HMS3369M05; HMS3651G07; HMS3713N10; HMS3747A17; HMS3872N13; Pharmakon1600-01500387; ACT11542; EBD16256; ZINC4658290; [S]C1=NC=NC2=C1NC=N2; Tox21_111158; Tox21_202591; 6450AJ; ANW-30999; ANW-57555; BBL033743; BDBM50423778; CCG-35344; CCG-39915; EBD540779; MFCD00233552; NSC759614; s1305; SBB037953; STK727062; STL257085; 6-Mercaptopurine, analytical standard; WLN: T56 BM DN FN HNJ ISH; AKOS000170222; AKOS000275858; AKOS005224624; AKOS008901311; AKOS016903205; Tox21_111158_1; AM81386; CCG-266232; CS-1499; DB01033; LS20858; MCULE-4811219863; NC00613; NSC-759614; IDI1_000493; NCGC00091641-02; NCGC00091641-03; NCGC00091641-04; NCGC00094717-01; NCGC00094717-02; NCGC00094717-03; NCGC00094717-05; NCGC00094717-06; NCGC00188973-01; NCGC00188973-02; NCGC00260139-01; AC-11464; AS-13109; H348; HY-13677; NCI60_041653; SMR004703503; ST086505; ST086506; SBI-0051437.P004; AB0013000; AB0119973; DB-026398; BB 0241023; FT-0621175; SW199090-2; EN300-61517; 1766-EP2270008A1; 1766-EP2272827A1; 1766-EP2272832A1; 1766-EP2277876A1; 1766-EP2292614A1; 1766-EP2292617A1; 1766-EP2295409A1; 1766-EP2295426A1; 1766-EP2295427A1; 1766-EP2298768A1; 1766-EP2298778A1; 1766-EP2308833A2; 1766-EP2308855A1; 1766-EP2308861A1; 1766-EP2314590A1; 1766-EP2316834A1; 1766-EP2374454A1; 50M442; C01756; C02380; D04931; W-5114; 15150-EP2272827A1; 15150-EP2275420A1; 15150-EP2295055A2; 15150-EP2295416A2; 15150-EP2298748A2; 15150-EP2298764A1; 15150-EP2298765A1; 15150-EP2305642A2; 15150-EP2305679A1; 15150-EP2308833A2; 15150-EP2311453A1; 15150-EP2311825A1; 15150-EP2311840A1; 15150-EP2311842A2; 15150-EP2316832A1; 15150-EP2316833A1; 21414-EP2270006A1; 21414-EP2270014A1; 21414-EP2272509A1; 21414-EP2272847A1; 21414-EP2275413A1; 21414-EP2277874A1; 21414-EP2284158A1; 21414-EP2287156A1; 21414-EP2287159A1; 21414-EP2295439A1; 21414-EP2311818A1; 21414-EP2314295A1; AB00171799_05; AB00641894-03; AB00641894-04; AB00641894_05; AB00876276-13; 233D552; 462M721; 599M524; A828129; Q418529; SR-05000001925-1; SR-05000001925-2; W-105961; purine antimetabolite: inhibits nucleic acid replication; 1,9-Dihydropurine-6-thione DISCONTINUED, see M225450; Mercaptopurine; 7H-Purine-6-thiol; Azathioprine BP Impurity B; 157930-11-5
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Mantle cell lymphoma [ICD-11: 2A85] | Approved | [1] | |
| Structure |
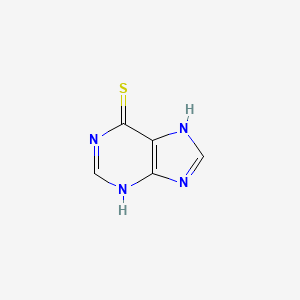
|
Click to Download Mol2D MOL |
||
| ADMET Property |
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 2: low solubility and high permeability
Clearance
The drug present in the plasma can be removed from the body at the rate of 15 mL/min/kg
Elimination
22% of drug is excreted from urine in the unchanged form
Half-life
The concentration or amount of drug in body reduced by one-half in 45 minutes, 2.5 hours
Half-life
The concentration or amount of drug in body reduced by one-half in 1 hour
Metabolism
The drug is metabolized via the hepatic
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 16.42808 micromolar/kg/day
Unbound Fraction
The unbound fraction of drug in plasma is 0.85%
Vd
Fluid volume that would be required to contain the amount of drug present in the body at the same concentration as in the plasma 1 L/kg
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C5H4N4S
|
|||
| PubChem CID | ||||
| Canonical SMILES |
C1=NC2=C(N1)C(=S)N=CN2
|
|||
| InChI |
1S/C5H4N4S/c10-5-3-4(7-1-6-3)8-2-9-5/h1-2H,(H2,6,7,8,9,10)
|
|||
| InChIKey |
GLVAUDGFNGKCSF-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 50-44-2
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Bismuth (III) | Homo sapiens | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Biological
Regulation |
Decrease | Cell proliferation capacity | ||||
| In-vitro Model | A-549 | CVCL_0023 | Lung adenocarcinoma | Homo sapiens | ||
| NCI-H460 | CVCL_0459 | Lung large cell carcinoma | Homo sapiens | |||
| Experimental
Result(s) |
The combination of Bi(III) with 6-MP endowed the newly developed amorphous [Bi(MP)3(NO3)2]NO3 with excellent anticancer activity against lung cancer cells and the solubility and bioavailability of the obtained [Bi(MP)3(NO3)2]NO3 were dramatically improved, compared with that of 6-MP. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Amidophosphoribosyltransferase (PPAT) | Molecule Info | [3] | |
| IMP dehydrogenase 1 (IMPDH1) | Molecule Info | [4] | ||
| BioCyc | Purine nucleotides degradation | Click to Show/Hide | ||
| 2 | Urate biosynthesis/inosine 5'-phosphate degradation | |||
| 3 | Guanosine nucleotides de novo biosynthesis | |||
| 4 | Superpathway of purine nucleotide salvage | |||
| 5 | Purine nucleotides de novo biosynthesis | |||
| 6 | Guanosine ribonucleotides de novo biosynthesis | |||
| KEGG Pathway | Purine metabolism | Click to Show/Hide | ||
| 2 | Drug metabolism - other enzymes | |||
| 3 | Metabolic pathways | |||
| Pathwhiz Pathway | Purine Metabolism | Click to Show/Hide | ||
| Reactome | Purine ribonucleoside monophosphate biosynthesis | Click to Show/Hide | ||
| WikiPathways | Nucleotide Metabolism | Click to Show/Hide | ||

