Drug Details
| General Information of the Drug (ID: DR5364) | ||||
|---|---|---|---|---|
| Name |
Alogliptin
|
|||
| Synonyms |
Alogliptin; 850649-61-5; UNII-JHC049LO86; Alogliptin (SYR-322); (R)-2-((6-(3-aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)benzonitrile; 2-({6-[(3r)-3-Aminopiperidin-1-Yl]-3-Methyl-2,4-Dioxo-3,4-Dihydropyrimidin-1(2h)-Yl}methyl)benzonitrile; JHC049LO86; CHEBI:72323; 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl}methyl)benzonitrile; alogliptina; vipidia; 2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl]benzonitrile; Alogliptin [INN]; alogliptine; alogliptinum; SYR322; HSDB 8203; SCHEMBL121028; CHEMBL376359; GTPL6319; BDBM16285; HY-A0023A; DTXSID90234130; AMY22119; Alogliptin pound SYR-322 pound(c); MFCD09833196; s2868; s5365; ZINC14961096; AKOS025149226; CCG-267914; CCG-267915; CS-1617; DB06203; SB20301; 2-((6-((3R)-3-aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl) benzonitrile; AC-26300; AS-19582; SW219186-1; Y1123; Q-4517; Q4734170; BRD-K83003151-057-02-4; 2-{6-[3(R)-Amino-piperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl}-benzonitrile
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Type 2 diabetes mellitus [ICD-11: 5A11] | Approved | [1] | |
| Structure |
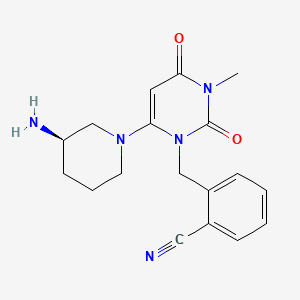
|
Click to Download Mol2D MOL |
||
| ADMET Property |
Clearance
The renal clearance of drug is 9.6 L/h
Half-life
The concentration or amount of drug in body reduced by one-half in 21 hours
Metabolism
The drug is not metabolised
Vd
The volume of distribution (Vd) of drug is 417 L
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C18H21N5O2
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CN1C(=O)C=C(N(C1=O)CC2=CC=CC=C2C#N)N3CCCC(C3)N
|
|||
| InChI |
1S/C18H21N5O2/c1-21-17(24)9-16(22-8-4-7-15(20)12-22)23(18(21)25)11-14-6-3-2-5-13(14)10-19/h2-3,5-6,9,15H,4,7-8,11-12,20H2,1H3/t15-/m1/s1
|
|||
| InChIKey |
ZSBOMTDTBDDKMP-OAHLLOKOSA-N
|
|||
| CAS Number |
CAS 850649-61-5
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Voglibose | Homo sapiens | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Alogliptin (0.03%) and voglibose (0.001%) alone or in combination were administered in the diet to prediabetic db/db mice. | |||||
| Experimental
Result(s) |
Chronic treatment with alogliptin in combination with voglibose concurrently increased active GLP-1 circulation, increased insulin secretion, decreased glucagon secretion, prevented the onset of the disease, and preserved pancreatic beta-cells and islet structure in prediabetic db/db mice. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Dipeptidyl peptidase 4 (DPP-4) | Molecule Info | [3] | |
| KEGG Pathway | Protein digestion and absorption | Click to Show/Hide | ||
| NetPath Pathway | IL2 Signaling Pathway | Click to Show/Hide | ||
| 2 | TGF_beta_Receptor Signaling Pathway | |||

