Drug Details
| General Information of the Drug (ID: DR6236) | ||||
|---|---|---|---|---|
| Name |
Nalidixic acid
|
|||
| Synonyms |
nalidixic acid; 389-08-2; Nalidixin; Nevigramon; Nalidixate; Uronidix; NegGram; Innoxalon; Nalidixan; Nalitucsan; Sicmylon; Unaserus; Nalidic acid; Nalidixinic acid; Wintomylon; Dixiben; Dixinal; Jicsron; Nalurin; Naxuril; Nogram; Urisal; Cybis; Nalix; 1-Ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid; Nalidicron; Betaxina; Kusnarin; Narigix; Nicelate; Specifen; Specifin; Uralgin; Uriclar; Urodixin; Negram; Poleon; Uriben; Uroman; Uroneg; Uropan; Acide nalidixique; Eucistin; Acide nalidixico; Acido nalidixico; Acidum nalidixicum; 1-Ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid; NSC-82174; Nalidixane; WIN 18,320; 1,4-Dihydro-1-ethyl-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid; 1-ethyl-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid; 3-Carboxy-1-ethyl-7-methyl-1,8-naphthyridin-4-one; NCI-C56199; MFCD00006884; 1,8-Naphthyridine-3-carboxylic acid, 1-ethyl-1,4-dihydro-7-methyl-4-oxo-; 3-Carboxy-1-ethyl-7-methyl-1,8-naphthidin-4-one; UNII-3B91HWA56M; 1-Ethyl-7-methyl-1,4-dihydro-1,8-naphthyridin-4-one-3-carboxylic acid; 1-Aethyl-7-methyl-1,8-naphthyridin-4-on-3-karbonsaeure; Nalidixic acid (NegGram); MLS000028504; 3B91HWA56M; Acide 1-etil-7-metil-1,8-naftiridin-4-one-3-carbossilico; Win 18320; CHEBI:100147; 1-Ethyl-7-methyl-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid; NSC82174; NCGC00018181-08; SMR000058264; Wintron; DSSTox_CID_912; 1,4-Dihydro-1-ethyl-7-methyl-1,8-naphthyridin-4-one-3-carboxylic acid; Acido nalidissico; Nalidixic acid, 99.5%; DSSTox_RID_75859; DSSTox_GSID_20912; Acido nalidissico [DCIT]; Acide nalidixico [Italian]; Acide nalidixique [French]; Nalidixic; Acide nalidixique [INN-French]; Acido nalidixico [INN-Spanish]; Acidum nalidixicum [INN-Latin]; 1,8-Naphthyridine-3-carboxylic acid,1-ethyl-1,4-dihydro-7-methyl-4-oxo-; CAS-389-08-2; NegGram (TN); CCRIS 2365; HSDB 3241; EINECS 206-864-7; BRN 0750515; Innoxalomn; Eucisten; nalidixic-acid; SR-01000003086; 1-Aethyl-7-methyl-1,8-naphthyridin-4-on-3-karbonsaeure [German]; Acide 1-etil-7-metil-1,8-naftiridin-4-one-3-carbossilico [Italian]; WIN-18320; WIN 183203; CHEMBL5; Spectrum_000918; Nalidixic acid [USAN:USP:INN:BAN:JAN]; Maybridge1_007101; Opera_ID_1064; Prestwick0_000187; Prestwick1_000187; Prestwick2_000187; Prestwick3_000187; Spectrum2_001360; Spectrum3_000075; Spectrum4_000817; Spectrum5_001540; Nalidixic acid, >=98%; UPCMLD-DP129; N-1200; NCIOpen2_004342; Lopac0_000837; Oprea1_010545; SCHEMBL21736; BSPBio_000113; BSPBio_001889; KBioGR_001333; KBioSS_001398; 5-25-07-00384 (Beilstein Handbook Reference); MLS001148578; MLS002303041; MLS004820190; MLS006011875; 1-ethyl-7-methyl-4-oxo-1; BIDD:GT0529; DivK1c_000058; SPECTRUM1500756; SPBio_001579; SPBio_002034; BPBio1_000125; DTXSID3020912; UPCMLD-DP129:001; BDBM21691; HMS500C20; HMS561K17; KBio1_000058; KBio2_001398; KBio2_003966; KBio2_006534; KBio3_001109; ZINC57421; NINDS_000058; HMS1921G10; HMS2092K04; HMS2232H24; HMS3259O13; HMS3374G11; HMS3656K05; Pharmakon1600-01500756; Nalidixic acid (JP17/USP/INN); Nalidixic acid, analytical standard; ALBB-021275; HY-B0398; Tox21_110835; Tox21_201477; Tox21_302754; ANW-42195; BBL012279; CCG-39298; NSC757432; SBB002146; STK735579; 1,8-Naphthyridine-3-carboxylicacid, 1-ethyl-1,4-dihydro-7-methyl-4-oxo-; 1-Ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxilic acid; AKOS000120074; Tox21_110835_1; DB00779; MCULE-6808033849; NC00494; NSC-757432; SDCCGSBI-0050814.P004; IDI1_000058; NCGC00018181-01; NCGC00018181-02; NCGC00018181-03; NCGC00018181-04; NCGC00018181-05; NCGC00018181-06; NCGC00018181-07; NCGC00018181-09; NCGC00018181-10; NCGC00018181-12; NCGC00018181-13; NCGC00021730-03; NCGC00021730-04; NCGC00021730-05; NCGC00021730-06; NCGC00021730-07; NCGC00256581-01; NCGC00259028-01; AK163550; AS-13289; H816; NCI60_041807; SMR004703506; WLN: T66 BN EV JNJ B2 DVQ I1; SBI-0050814.P003; AB0013282; DB-049349; 1, 1-ethyl-1,4-dihydro-7-methyl-4-oxo-; BB 0242389; FT-0603390; N0490; S2328; SW219624-1; 1-Ethyl-1,8-naphthyridine-3-carboxilic acid; 1-Ethyl-1,8-naphthyridine-3-carboxylic acid; Nalidixic acid 100 microg/mL in Acetonitrile; VU0239598-6; C05079; D00183; K-8443; Nalidixic acid, meets USP testing specifications; 389N082; Q281082; SR-01000003086-4; SR-01000003086-6; BRD-K47886988-323-03-0; Nalidixic acid, Antibiotic for Culture Media Use Only; SR-01000003086-10; F0850-6751; Z256708444; 1-Ethyl-7-methyl-1,8-naphthyridin-4-one-3-carboxylic acid; 1-ethyl-7-methyl-4-oxo-[1,8]naphthyridine-3-carboxylic acid; Nalidixic acid, European Pharmacopoeia (EP) Reference Standard; Nalidixic acid, United States Pharmacopeia (USP) Reference Standard; 1-Ethyl-7-methyl-4-oxo-1,4-dihydro-[1,8]naph thyridine-3-carboxylic acid; 1-ethyl-7-methyl-4-oxohydropyridino[2,3-b]pyridine-3-carboxylic acid; N-benzyl-4-[(5-cyclobutyl-1,2,4-oxadiazol-3-yl)methyl]-N-ethyl-3-oxo-3,4-dihydro-2H-1,4-benzoxazine-6-sulfonamide
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Urinary tract infection [ICD-11: GC08] | Approved | [1] | |
| Structure |
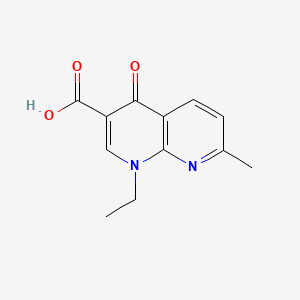
|
Click to Download Mol2D MOL |
||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C12H12N2O3
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CCN1C=C(C(=O)C2=C1N=C(C=C2)C)C(=O)O
|
|||
| InChI |
1S/C12H12N2O3/c1-3-14-6-9(12(16)17)10(15)8-5-4-7(2)13-11(8)14/h4-6H,3H2,1-2H3,(H,16,17)
|
|||
| InChIKey |
MHWLWQUZZRMNGJ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 389-08-2
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Camellia sinensis | Camellia sinensis | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vitro Model | Staphylococcus aureus | Microorganism model | Staphylococcus aureus | |||
| Streptococcus pyogenes | Microorganism model | Streptococcus pyogenes | ||||
| Escherichia coli | Microorganism model | Escherichia coli | ||||
| Salmonella enterica serovar Typhi | Microorganism model | Salmonella enterica | ||||
| Shigella species | Microorganism model | Shigella | ||||
| Salmonella Paratyphi A | Microorganism model | Salmonella Paratyphi | ||||
| Pseudomonas aeuginosa | Microorganism model | Pseudomonas aeruginosa | ||||
| Acinetobacter baumannii | Microorganism model | Acinetobacter baumannii | ||||
| Klebseilla pneumoniae | Microorganism model | Klebseilla pneumoniae | ||||
| Citrobacter freuendii | Microorganism model | Citrobacter freuendii | ||||
| Enterobacter cloacae | Microorganism model | Enterobacter cloacae | ||||
| Bacillus subtilis | Microorganism model | Bacillus subtilis | ||||
| Streptococcus pneumoniae | Microorganism model | Streptococcus pneumoniae | ||||
| Micrococcus | Microorganism model | Micrococcus | ||||
| Helicobacter pylori | Microorganism model | Helicobacter pylori | ||||
| Campylobacter jejuni | Microorganism model | Campylobacter jejuni | ||||
| Pasteurella multocida | Microorganism model | Pasteurella multocida | ||||
| Experimental
Result(s) |
When Camellia sinensis was combined with nalidixic acid, it was able to inhibit S. Typhi at sub-MIC levels. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II (TOP2) | Molecule Info | [3] | |

