Drug Details
| General Information of the Drug (ID: DR6343) | ||||
|---|---|---|---|---|
| Name |
Lobeline
|
|||
| Synonyms |
lobeline; alpha-Lobeline; (-)-Lobeline; Lobnico; Lobelin; 8,10-Diphenyllobelionol; Lobelina; Lobelinum; 90-69-7; Inflatine; UNII-D0P25S3P81; 2-(6-(2-Hydroxy-2-phenylethyl)-1-methyl-2-piperidinyl)-1-phenylethanone; 2-{(2R,6S)-6-[(2S)-2-hydroxy-2-phenylethyl]-1-methylpiperidin-2-yl}-1-phenylethanone; CHEMBL122270; CHEBI:48723; 2-(6-(beta-Hydroxyphenethyl)-1-methyl-2-piperidyl)acetophenone; D0P25S3P81; NCGC00024378-05; Alpha-Lobeline HCl; Lobeline [INN:BAN]; Lobelinum [INN-Latin]; Lobelina [INN-Spanish]; SR-01000075960; Lobeline A; Lobeline (INN); L0B; EINECS 202-012-3; BRN 0091533; Prestwick0_000585; Prestwick1_000585; Prestwick2_000585; Prestwick3_000585; DSSTox_CID_3219; DSSTox_RID_76929; DSSTox_GSID_23219; Lopac0_000698; BSPBio_000430; 5-21-12-00627 (Beilstein Handbook Reference); Ethanone, 2-(6-(2-hydroxy-2-phenylethyl)-1-methyl-2-piperidinyl)-1-phenyl-, (2R-(2alpha,6alpha(S*)))-; SCHEMBL290803; SPBio_002649; BPBio1_000474; ZINC1624; DTXSID3023219; Tox21_110900; BDBM50080818; AKOS024278800; CCG-204783; DB05137; NSC 757421; SDCCGSBI-0050676.P002; 2-((2R,6S)-6-((S)-2-hydroxy-2-phenylethyl)-1-methylpiperidin-2-yl)-1-phenylethan-1-one; CAS-90-69-7; NCGC00024378-06; NCGC00024378-07; NCGC00024378-08; NCGC00024378-09; NCGC00024378-10; NCGC00024378-14; AB00489926; C07475; D02364; Q421905; SR-01000075960-5; BRD-K66206289-003-03-1; BRD-K66206289-003-09-8; UNII-73BJ3LA259 component MXYUKLILVYORSK-HBMCJLEFSA-N
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Unspecific substance use disorder [ICD-11: 6C4Z] | Phase 2 | [1] | |
| Structure |
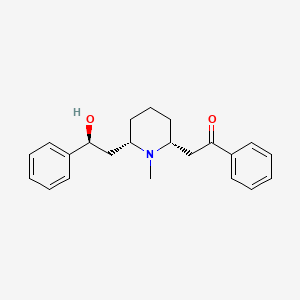
|
Click to Download Mol2D MOL |
||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C22H27NO2
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CN1C(CCCC1CC(=O)C2=CC=CC=C2)CC(C3=CC=CC=C3)O
|
|||
| InChI |
1S/C22H27NO2/c1-23-19(15-21(24)17-9-4-2-5-10-17)13-8-14-20(23)16-22(25)18-11-6-3-7-12-18/h2-7,9-12,19-21,24H,8,13-16H2,1H3/t19-,20+,21-/m0/s1
|
|||
| InChIKey |
MXYUKLILVYORSK-HBMCJLEFSA-N
|
|||
| CAS Number |
CAS 90-69-7
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Methyltransferase-like protein 3 (METTL3) | Molecule Info | ||
| Synaptic vesicle amine transporter (SLC18A2) | Molecule Info | [2] | ||
| KEGG Pathway | Synaptic vesicle cycle | Click to Show/Hide | ||
| 2 | Serotonergic synapse | |||
| 3 | Dopaminergic synapse | |||
| 4 | Parkinson's disease | |||
| 5 | Cocaine addiction | |||
| 6 | Amphetamine addiction | |||
| 7 | Alcoholism | |||
| Panther Pathway | Adrenaline and noradrenaline biosynthesis | Click to Show/Hide | ||
| 2 | 5HT1 type receptor mediated signaling pathway | |||
| 3 | 5HT2 type receptor mediated signaling pathway | |||
| 4 | 5HT3 type receptor mediated signaling pathway | |||
| 5 | 5HT4 type receptor mediated signaling pathway | |||
| 6 | Dopamine receptor mediated signaling pathway | |||
| 7 | Nicotine pharmacodynamics pathway | |||
| 8 | CCKR signaling map ST | |||
| Reactome | Norepinephrine Neurotransmitter Release Cycle | Click to Show/Hide | ||
| 2 | Na+/Cl- dependent neurotransmitter transporters | |||
| WikiPathways | Dopaminergic Neurogenesis | Click to Show/Hide | ||
| 2 | Synaptic Vesicle Pathway | |||
| 3 | Neurotransmitter Release Cycle | |||
| 4 | Nicotine Activity on Dopaminergic Neurons | |||
