Drug Details
| General Information of the Drug (ID: DR9285) | ||||
|---|---|---|---|---|
| Name |
Diltiazem
|
|||
| Synonyms |
diltiazem; 42399-41-7; d-cis-Diltiazem; Cardizem; Dilt-cd; Diltiazemum; Anoheal; Diltzac; Tiamate; (+)-diltiazem; Cardil; Dilren; Tiazac; (+)-cis-diltiazem; Diltiazen; UNII-EE92BBP03H; Diltiazem free base; CRD-401; (2S,3S)-5-(2-(dimethylamino)ethyl)-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepin-3-yl acetate; CHEMBL23; Dilcontin; Dilticard; Endrydil; Acalix; Dilzen; Dilta-Hexal; EE92BBP03H; Incoril AP; CHEBI:101278; 42399-41-7 (free base); (2S,3S)-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl acetate; [(2S,3S)-5-(2-dimethylaminoethyl)-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepin-3-yl] acetate; Acetic acid (2S,3S)-5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl ester; NCGC00024309-05; DSSTox_CID_2940; Diltiazem [INN:BAN]; Dilacor-XR; DSSTox_RID_76797; Diltiazemum [INN-Latin]; DSSTox_GSID_22940; (+)-cis-5-[2-(dimethylamino)ethyl]-2,3-dihydro-3-hydroxy-2-(p-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one acetate ester; (2S-cis)-3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one; Diltiazem (INN); [(2~{S},3~{S})-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxidanylidene-2,3-dihydro-1,5-benzothiazepin-3-yl] ethanoate; Surazem (TN); CAS-42399-41-7; HSDB 6528; Cardizem (Hydrochloride); MK-793 (Malate); RG-83606; EINECS 255-796-4; BRN 3573079; tetrahydrobenzo; RG 83606 (Hydrochloride); Diltiazem HCI; D-(cis)-diltiazem; Tocris-0685; 103532-26-9; Prestwick0_000134; Prestwick1_000134; Prestwick2_000134; Prestwick3_000134; Lopac0_000327; SCHEMBL17776; BSPBio_000208; BSPBio_001311; LITHIUMDICYCLOHEXYLAMIDE; BIDD:GT0548; SPBio_002147; BPBio1_000230; GTPL2298; DTXSID9022940; Bio1_000371; Bio1_000860; Bio1_001349; HMS1791B13; HMS1989B13; HMS2089H09; ZINC621893; [b][1,4]thiazepin-3-yl acetate; Acetic acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl ester; methoxyphenyl)-4-oxo-2,3,4,5-; Tox21_110898; AC-936; BDBM50004704; CD0163; AKOS015897257; Tox21_110898_1; CCG-204422; DB00343; MCULE-9931403933; SDCCGSBI-0050315.P002; VA10739; NCGC00024309-02; NCGC00024309-04; NCGC00024309-06; NCGC00024309-07; NCGC00024309-08; NCGC00024309-09; NCGC00024309-10; NCGC00024309-11; NCGC00024309-17; NCGC00024309-21; NCGC00024309-27; (2S,3S)-5-[2-(dimethylamino)ethyl]-2-[4-(methyloxy)phenyl]-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl acetate; [(2S,3S)-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepin-3-yl] acetate; 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, (2S,3S)-; C06958; D07845; (2S,3S)-5-(2-(dimethylamino)ethyl)-2-(4-; Q422229; W-106274; BRD-K24023109-001-02-5; BRD-K24023109-003-03-9; BRD-K24023109-003-11-2; BRD-K24023109-003-20-3; 8-Chloro-1-methyl-6-phenyl-4H-2,3,5,10b-tetraaza-benzo[e]azulene; 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl ester(Diltiazem)Acetic acid; Acetic acid (S)-5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl ester; Acetic acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl ester (Diltiazem); Acetic acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl ester(cis-(+)-Diltiazem); Acetic acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl ester; hydrochloride; C9F; cis-Acetic acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl ester; diltiazem;Acetic acid 5-(2-dimethylamino-ethyl)-2-(4-methoxy-phenyl)-4-oxo-2,3,4,5-tetrahydro-benzo[b][1,4]thiazepin-3-yl ester
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Hypertension [ICD-11: BA00] | Approved | [1] | |
| Structure |
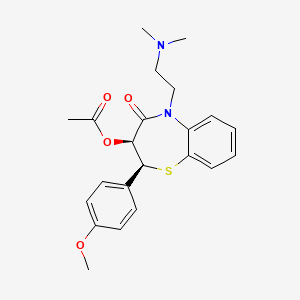
|
Click to Download Mol2D MOL |
||
| ADMET Property |
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 1: high solubility and high permeability
Bioavailability
The bioavailability of drug is 40%
Clearance
The sytemic clearance of drug is 65 L/h
Elimination
Due to its extensive metabolism, only 2% to 4% of the unchanged drug can be detected in the urine
Half-life
The concentration or amount of drug in body reduced by one-half in 3.0 - 4.5 hours
Metabolism
The drug is metabolized via the CYP3A4
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 19.29908 micromolar/kg/day
Unbound Fraction
The unbound fraction of drug in plasma is 0.18%
Vd
The volume of distribution (Vd) of drug is 305 L
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C22H26N2O4S
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CC(=O)OC1C(SC2=CC=CC=C2N(C1=O)CCN(C)C)C3=CC=C(C=C3)OC
|
|||
| InChI |
1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1
|
|||
| InChIKey |
HSUGRBWQSSZJOP-RTWAWAEBSA-N
|
|||
| CAS Number |
CAS 56209-45-1
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Calcium channel alpha-2/delta-1 (CACNA2D1) | Molecule Info | [2] | |
| KEGG Pathway | MAPK signaling pathway | Click to Show/Hide | ||
| 2 | Cardiac muscle contraction | |||
| 3 | Adrenergic signaling in cardiomyocytes | |||
| 4 | Oxytocin signaling pathway | |||
| 5 | Hypertrophic cardiomyopathy (HCM) | |||
| 6 | Arrhythmogenic right ventricular cardiomyopathy (ARVC) | |||
| 7 | Dilated cardiomyopathy | |||
| Panther Pathway | Muscarinic acetylcholine receptor 2 and 4 signaling pathway | Click to Show/Hide | ||
| WikiPathways | Arrhythmogenic Right Ventricular Cardiomyopathy | Click to Show/Hide | ||
| 2 | miR-targeted genes in muscle cell - TarBase | |||
| 3 | miR-targeted genes in lymphocytes - TarBase | |||
