Natural Product (NP) Details
| General Information of the NP (ID: NP0165) | |||||
|---|---|---|---|---|---|
| Name |
Voglibose
|
||||
| Synonyms |
voglibose; 83480-29-9; Glustat; Basen; AO-128; UNII-S77P977AG8; A-71100; Voglibosa; CHEMBL476960; (1S,2S,3R,4S,5S)-5-((1,3-dihydroxypropan-2-yl)amino)-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetraol; S77P977AG8; AO 128; DSSTox_CID_1442; DSSTox_RID_76161; DSSTox_GSID_21442; (1S,2S,3R,4S,5S)-5-(1,3-dihydroxypropan-2-ylamino)-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetraol; (1S,2S,3R,4S,5S)-5-[(1,3-dihydroxypropan-2-yl)amino]-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetrol; Voglibosum; 3,4-Dideoxy-4-((2-hydroxy-1-(hydroxymethyl)ethyl)amino)-2-C-(hydroxymethyl)-D-epi-inositol; Basen OD; 3,4-DIDEOXY-4-[[2-HYDROXY-1-(HYDROXYMETHYL)ETHYL]AMINO]-2-C-(HYDROXYMETHYL)-D-EPINOSITOL; CAS-83480-29-9; Basen (TN); Voglibose [USAN:INN]; Voglibosum [INN-Latin]; Voglibosa [INN-Spanish]; CCRIS 4540; Voglibose/; NCGC00164595-01; 3,4-Dideoxy-4-[[2-hydroxy-1-(hydroxymethyl)ethyl]amino]-2-C-(hydroxymethyl)-D-epi-inositol; VOG; SCHEMBL5882; A 71100; MLS003882582; Voglibose (JP17/USAN/INN); DTXSID2021442; CHEBI:32300; AOB5593; BCPP000020; HMS3414A17; HMS3678A17; Voglibose, >=97.0% (TLC); HY-B0025; ZINC3788703; Tox21_112220; ABP000769; BDBM50263044; s4101; AKOS015950839; Tox21_112220_1; CCG-267119; DB04878; NCGC00164595-02; (1S,2S,3R,4S,5S)-5-{[2-hydroxy-1-(hydroxymethyl)ethyl]amino}-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetrol; SMR002530327; D01665; AB01566929_01; 480V299; SR-01000883931; Q-101310; Q7939403; SR-01000883931-1; BRD-K66850609-001-01-7; BRD-K66850609-001-07-4
Click to Show/Hide
|
||||
| Species Origin | Homo sapiens ... | Click to Show/Hide | |||
| Homo sapiens | |||||
| Disease | Acute diabete complication [ICD-11: 5A2Y] | Approved | [1] | ||
| Structure |
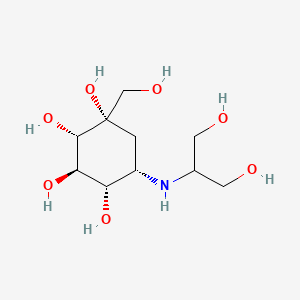
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-6.198
MDCK Permeability
-4.123
PAMPA
+++
HIA
+++
Distribution
VDss
-0.509
PPB
24.1%
BBB
- - -
Metabolism
CYP1A2 inhibitor
- - -
CYP1A2 substrate
- - -
CYP2C19 inhibitor
- - -
CYP2C19 substrate
- - -
CYP2C9 inhibitor
- - -
CYP2C9 substrate
- -
CYP2D6 inhibitor
- - -
CYP2D6 substrate
- - -
CYP3A4 inhibitor
- - -
CYP3A4 substrate
- - -
CYP2B6 inhibitor
- - -
CYP2B6 substrate
- - -
CYP2C8 inhibitor
- - -
HLM Stability
- - -
Excretion
CLplasma
2.249
T1/2
2.237
Toxicity
DILI
- - -
Rat Oral Acute Toxicity
- - -
FDAMDD
- - -
Respiratory
- - -
Human Hepatotoxicity
+
Ototoxicity
+++
Drug-induced Nephrotoxicity
++
Drug-induced Neurotoxicity
- - -
Hematotoxicity
- -
Genotoxicity
- - -
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C10H21NO7
|
||||
| PubChem CID | |||||
| Canonical SMILES |
C1C(C(C(C(C1(CO)O)O)O)O)NC(CO)CO
|
||||
| InChI |
1S/C10H21NO7/c12-2-5(3-13)11-6-1-10(18,4-14)9(17)8(16)7(6)15/h5-9,11-18H,1-4H2/t6-,7-,8+,9-,10-/m0/s1
|
||||
| InChIKey |
FZNCGRZWXLXZSZ-CIQUZCHMSA-N
|
||||
| CAS Number |
CAS 83480-29-9
|
||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Alogliptin | Type 2 diabetes mellitus | Click to Show/Hide the Molecular Data of This Drug | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Alogliptin (0.03%) and voglibose (0.001%) alone or in combination were administered in the diet to prediabetic db/db mice. | |||||
| Experimental
Result(s) |
Chronic treatment with alogliptin in combination with voglibose concurrently increased active GLP-1 circulation, increased insulin secretion, decreased glucagon secretion, prevented the onset of the disease, and preserved pancreatic beta-cells and islet structure in prediabetic db/db mice. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Intestinal maltase-glucoamylase (MGAM) | Molecule Info | [3] | |
| KEGG Pathway | Galactose metabolism | Click to Show/Hide | ||
| 2 | Starch and sucrose metabolism | |||
| 3 | Metabolic pathways | |||
| 4 | Carbohydrate digestion and absorption | |||
| Pathwhiz Pathway | Starch and Sucrose Metabolism | Click to Show/Hide | ||
| WikiPathways | Metabolism of carbohydrates | Click to Show/Hide | ||

