| General Information of the NP (ID: NP0862) |
| Name |
Glabratephrin
|
| Synonyms |
Glabratephrin
(+)-GLABRATEPHRIN
CHEMBL2286767
LMPK12110014
[(3'S,9R)-2',2'-dimethyl-4,5'-dioxo-2-phenylspiro[8H-furo[2,3-h]chromene-9,4'-oxolane]-3'-yl] acetate
Click to Show/Hide
|
| Species Origin |
. ...
|
Click to Show/Hide
|
| . |
SuperKingdom: Eukaryota
Kingdom: Viridiplantae
Phylum: Tracheophyta
Class: Magnoliopsida
Order: Fabales
Family: Fabaceae
Genus: Tephrosia
Species: Tephrosia glabra
|
| Disease |
Breast cancer
[ICD-11: 2C60] |
Investigative |
|
| Structure |
|
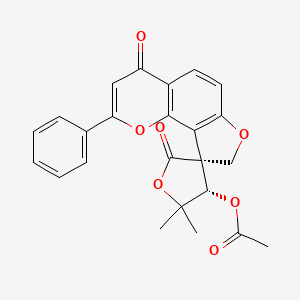
|
Click to Download Mol
2D MOL
3D MOL
|
| ADMET Property |
Absporption
Caco-2 Permeability
-4.854
MDCK Permeability
-4.555
Distribution
Metabolism
CYP1A2 inhibitor
++
CYP1A2 substrate
- - -
CYP2C19 inhibitor
+++
CYP2C19 substrate
- -
CYP2C9 inhibitor
- -
CYP2C9 substrate
+++
CYP2D6 inhibitor
- - -
CYP2D6 substrate
- -
CYP3A4 inhibitor
++
CYP3A4 substrate
- - -
CYP2B6 inhibitor
+++
CYP2B6 substrate
- - -
CYP2C8 inhibitor
+++
HLM Stability
++
Excretion
CLplasma
3.401
T1/2
1.167
Toxicity
DILI
+++
Rat Oral Acute Toxicity
+
Human Hepatotoxicity
+
Ototoxicity
- -
Drug-induced Nephrotoxicity
+
Drug-induced Neurotoxicity
-
Hematotoxicity
-
Genotoxicity
+++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
|
Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product
|
| Formula |
C24H20O7
|
| PubChem CID |
|
| Canonical SMILES |
CC(=O)O[C@H]1[C@@]2(COC3=C2C4=C(C=C3)C(=O)C=C(O4)C5=CC=CC=C5)C(=O)OC1(C)C
|
| InChI |
InChI=1S/C24H20O7/c1-13(25)29-21-23(2,3)31-22(27)24(21)12-28-17-10-9-15-16(26)11-18(30-20(15)19(17)24)14-7-5-4-6-8-14/h4-11,21H,12H2,1-3H3/t21-,24+/m1/s1
|
| InChIKey |
QSNBHLXYLHVCLT-QPPBQGQZSA-N
|