Natural Product (NP) Details
| General Information of the NP (ID: NP3689) | |||||
|---|---|---|---|---|---|
| Name |
Carnosine
|
||||
| Synonyms |
L-Carnosine; Carnosine; 305-84-0; beta-Alanyl-L-histidine; Ignotine; Karnozin; Karnozzn; L-Histidine, beta-alanyl-; L-Ignotine; N-2-M; UNII-8HO6PVN24W; Polaprezinc; CHEBI:15727; Nalpha-(beta-alanyl)-L-histidine; L-HISTIDINE, N-beta-ALANYL-; MFCD00005207; NSC 524045; BRN 0087671; 8HO6PVN24W; (3-aminopropanoyl)-L-histidine; L-Histidine, N-.beta.-alanyl-; beta-Alanylhistidine; L-Carnosine, 98%; carnosine zwitterion; Z-103; Z 103; N-beta-alanyl-L-histidine; (2S)-2-(3-aminopropanamido)-3-(1H-imidazol-4-yl)propanoic acid; (2S)-2-(3-aminopropanoylamino)-3-imidazol-4-ylpropanoic acid; (2~{S})-2-(3-azanylpropanoylamino)-3-(1~{H}-imidazol-4-yl)propanoic acid; .beta.-Alanyl-L-histidine; Sevitin; betaAla-His; b-Alanylhistidine; beta-Ala-His-OH; EINECS 206-169-9; 3-Chloromandelicacid; b-Alanyl-L-histidine; PubChem6017; beta-alanyl-l-histidin; .beta.-Alanylhistidine; Spectrum_001178; N-b-alanyl-L-Histidine; SpecPlus_000374; Spectrum2_000454; Spectrum3_001212; Spectrum4_001673; Spectrum5_000605; 3-aminopropionyl-histidine; bmse000246; bmse001002; C00386; N-(b-Alanyl)-L-histidine; SCHEMBL33769; BSPBio_002624; KBioGR_002225; KBioSS_001658; 4-25-00-04381 (Beilstein Handbook Reference); DivK1c_006470; SPECTRUM1500944; N-(3-Aminopropanoyl)histidine; SPBio_000528; CHEMBL242948; GTPL4559; KBio1_001414; KBio2_001658; KBio2_004226; KBio2_006794; KBio3_002124; DTXSID80879594; L-Carnosine, ~99%, crystalline; TNP00340; ZINC2040854; N(alpha)-(beta-alanyl)-L-histidine; ANW-26923; BDBM50485554; CCG-38696; SBB003316; STL466172; AKOS010421481; AKOS015963345; CS-W014210; DB11695; HY-W013494; MCULE-5739145561; SDCCGMLS-0066726.P001; NCGC00017390-01; NCGC00017390-02; NCGC00142487-01; AC-17084; AC-19690; AC-31940; AS-12570; ST057076; A0222; L-Carnosine, Vetec(TM) reagent grade, 98%; S5226; 305C840; Q413822; SR-05000002473; SR-05000002473-1; (2S)-2-(3-aminopropanamido)-3-(1H-imidazol-5-yl)propanoic acid; (S)-2-(3-aminopropanamido)-3-(1H-imidazol-4-yl)propanoic acid; 8V0
Click to Show/Hide
|
||||
| Species Origin | Homo sapiens ... | Click to Show/Hide | |||
| Homo sapiens | |||||
| Disease | Diabetic retinopathy [ICD-11: 9B71] | Investigative | [1] | ||
| Structure |
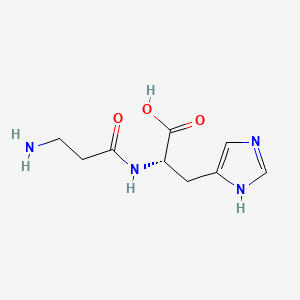
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-6.398
MDCK Permeability
-5.537
PAMPA
+++
HIA
-
Distribution
VDss
-0.394
PPB
7.1%
BBB
- - -
Metabolism
CYP1A2 inhibitor
- - -
CYP1A2 substrate
- - -
CYP2C19 inhibitor
- - -
CYP2C19 substrate
- - -
CYP2C9 inhibitor
- - -
CYP2C9 substrate
- -
CYP2D6 inhibitor
- - -
CYP2D6 substrate
- - -
CYP3A4 inhibitor
- - -
CYP3A4 substrate
- - -
CYP2B6 inhibitor
- - -
CYP2B6 substrate
- - -
CYP2C8 inhibitor
- - -
HLM Stability
- - -
Excretion
CLplasma
2.062
T1/2
1.696
Toxicity
DILI
- -
Rat Oral Acute Toxicity
-
FDAMDD
- -
Respiratory
-
Human Hepatotoxicity
- -
Ototoxicity
-
Drug-induced Nephrotoxicity
-
Drug-induced Neurotoxicity
-
Hematotoxicity
- - -
Genotoxicity
+
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C9H14N4O3
|
||||
| PubChem CID | |||||
| Canonical SMILES |
C1=C(NC=N1)CC(C(=O)O)NC(=O)CCN
|
||||
| InChI |
1S/C9H14N4O3/c10-2-1-8(14)13-7(9(15)16)3-6-4-11-5-12-6/h4-5,7H,1-3,10H2,(H,11,12)(H,13,14)(H,15,16)/t7-/m0/s1
|
||||
| InChIKey |
CQOVPNPJLQNMDC-ZETCQYMHSA-N
|
||||
| CAS Number |
CAS 305-84-0
|
||||
| ChEBI ID | |||||
| Herb ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Lisinopril | Hypertension | Click to Show/Hide the Molecular Data of This Drug | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Male Sprague-Dawley rats were injected i.v. with streptozotocin (STZ) to induce diabetes. | |||||
| Experimental
Result(s) |
Both carnosine and lisinopril exert distinct beneficial effects in a standard model of diabetic nephropathy. Both drugs administered together combine the respective effects of single treatment, albeit without exerting additive nephroprotection. | |||||

