Natural Product (NP) Details
| General Information of the NP (ID: NP4339) | |||||
|---|---|---|---|---|---|
| Name |
Aclarubicin
|
||||
| Synonyms |
aclarubicin; Aclacinomycin A; 57576-44-0; Aclarubicine; Aclarubicinum; NSC-208734; UNII-74KXF8I502; MA 144-A1; Aclucinomycin A; 74KXF8I502; Aclacinon; Aclacur; Jaclacin; methyl (1R,2R,4S)-4-[(2R,4S,5S,6S)-4-(dimethylamino)-5-[(2S,4S,5S,6S)-4-hydroxy-6-methyl-5-[(2R,6S)-6-methyl-5-oxooxan-2-yl]oxyoxan-2-yl]oxy-6-methyloxan-2-yl]oxy-2-ethyl-2,5,7-trihydroxy-6,11-dioxo-3,4-dihydro-1H-tetracene-1-carboxylate; Aclarubicino; Aclarubicins; Aclacin; CHEBI:77980; ACLACINOMYCIN A1; methyl (1R,2R,4S)-2-ethyl-2,5,7-trihydroxy-6,11-dioxo-4-{[2,3,6-trideoxy-4-O-{2,6-dideoxy-4-O-[(2R,6S)-6-methyl-5-oxotetrahydro-2H-pyran-2-yl]-alpha-L-lyxo-hexopyranosyl}-3-(dimethylamino)-alpha-L-lyxo-hexopyranosyl]oxy}-1,2,3,4,6,11-hexahydrotetracene-1-carboxylate; Antibiotic MA 144A; Aclarubicine [INN-French]; Aclarubicinum [INN-Latin]; Aclarubicino [INN-Spanish]; aclarubicina; Aclarubicin [USAN:INN:BAN]; CCRIS 2262; EINECS 260-824-3; Spectrum_000467; Spectrum2_001934; Spectrum4_000768; Spectrum5_001041; Aclarubicin (USAN/INN); SCHEMBL4532; KBioGR_001156; KBioSS_000947; SPBio_001967; CHEMBL502620; DTXSID1022554; CHEBI:74619; KBio2_000947; KBio2_003515; KBio2_006083; HMS2089B13; AW8655; BDBM50368351; CCG-39864; MFCD00866250; ZINC85537142; DB11617; 1-Naphthacenecarboxylic acid,(2-ethyl-1,2,3,4,6,11-hexahydro-2,5,7-trihydroxy-6,11-dioxo-4-((2,3,6-trideoxy-4-O-(2,6-dideoxy-4-O-((2R-trans)-tetrahydro-6-methyl-5-oxo-2H-pyran-2-yl)-alpha-L-lyxo-hexopyranosyl)-3-(dimethylamino)-alpha-L-lyxo-hexopyranosyl)-3-(dimethylamino)-alpha-L-Lyxo-hexopyranosyl)oxy)-, methyl ester, (1R-(1alpha,2beta,4beta))-; Methyl (1R,2R,4S)-2-ethyl-1,2,3,4,6,11-hexahydro-2,5,7-trihydroxy-6,11-dioxo-4-((2,3,6-trideoxy-4-O-(2,6-dideoxy-4-O-((2R,6S)-tetrahydro-6-methyl-5-oxo-2H-pyran-2-yl)-alpha-L-lyxo-hexopyranosyl)-3-(dimethylamino)-alpha-L-lyxo-hexopyranosyl)oxy)-1-naphthacenecarboxylate; C18638; D02756; AB00052311-02; 576A440; Q4674302; 1-Naphthacenecarboxylic acid, 2-ethyl-1,2,3,4,6,11-hexahydro-2,5,7-trihydroxy-6,11-dioxo-4-((2,3,6-trideoxy-4-O-(2,6-dideoxy-4-O-((2R-trans)-tetrahydro-6-methyl-5-oxo-2H-pyran-2-yl)-alpha-L-lyxo-hexopyranosyl)-3-(dimethylamino)-alpha-L-lyxo-hexopyranosyl)oxy)-, methyl ester, (1R-(1alpha,2beta,4beta))-; 2-Ethyl-1,2,3,4,6,11-hexahydro-2,5,7-trihydroxy-6,11-dioxo-4-[[2,3,6-trideoxy-4-O-[2,6-dideoxy-4-O-[(2R-trans)-tetrahydro-6-methyl-5-oxo-2H-pyran-2-yl]-.alpha.-L-lyxo-hexopyranosyl]-3-(dimethylamino)-.alpha.-L-lyxo-hexopyranosyl]-oxy]-1-naphthacenecarboxalic acid methyl ester; methyl (1R,2R,4S)-4-[(2R,4S,5S,6S)-4-(dimethylamino)-5-[(2S,4S,5S,6S)-4-hydroxy-6-methyl-5-[(2R,6S)-6-methyl-5-oxo-tetrahydropyran-2-yl]oxy-tetrahydropyran-2-yl]oxy-6-methyl-tetrahydropyran-2-yl]oxy-2-ethyl-2,5,7-trihydroxy-6,11-dioxo-3,4-dihydro-1H-tetracene-1-carboxylate
Click to Show/Hide
|
||||
| Species Origin | Streptomyces galilaeus ... | Click to Show/Hide | |||
| Streptomyces galilaeus | |||||
| Disease | Acute myeloid leukemia [ICD-11: 2A60] | Approved | [1] | ||
| Structure |
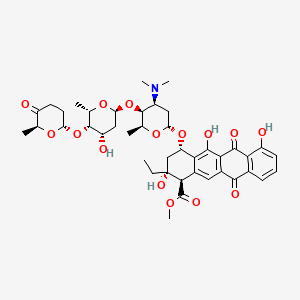
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-6.252
MDCK Permeability
-4.977
PAMPA
+++
HIA
- - -
Distribution
VDss
0.19
PPB
83.6%
BBB
- - -
Metabolism
CYP1A2 inhibitor
- - -
CYP1A2 substrate
- - -
CYP2C19 inhibitor
- - -
CYP2C19 substrate
- -
CYP2C9 inhibitor
- - -
CYP2C9 substrate
- - -
CYP2D6 inhibitor
- - -
CYP2D6 substrate
- - -
CYP3A4 inhibitor
- - -
CYP3A4 substrate
++
CYP2B6 inhibitor
- -
CYP2B6 substrate
- - -
CYP2C8 inhibitor
- - -
HLM Stability
- - -
Excretion
CLplasma
4.913
T1/2
4.465
Toxicity
DILI
+++
Rat Oral Acute Toxicity
+++
FDAMDD
+++
Respiratory
+++
Human Hepatotoxicity
+++
Ototoxicity
+++
Drug-induced Nephrotoxicity
+++
Drug-induced Neurotoxicity
+++
Hematotoxicity
+++
Genotoxicity
+++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C42H53NO15
|
||||
| PubChem CID | |||||
| Canonical SMILES |
CCC1(CC(C2=C(C3=C(C=C2C1C(=O)OC)C(=O)C4=C(C3=O)C(=CC=C4)O)O)OC5CC(C(C(O5)C)OC6CC(C(C(O6)C)OC7CCC(=O)C(O7)C)O)N(C)C)O
|
||||
| InChI |
1S/C42H53NO15/c1-8-42(51)17-28(33-22(35(42)41(50)52-7)14-23-34(38(33)49)37(48)32-21(36(23)47)10-9-11-26(32)45)56-30-15-24(43(5)6)39(19(3)54-30)58-31-16-27(46)40(20(4)55-31)57-29-13-12-25(44)18(2)53-29/h9-11,14,18-20,24,27-31,35,39-40,45-46,49,51H,8,12-13,15-17H2,1-7H3/t18-,19-,20-,24-,27-,28-,29-,30-,31-,35-,39+,40+,42+/m0/s1
|
||||
| InChIKey |
USZYSDMBJDPRIF-SVEJIMAYSA-N
|
||||
| CAS Number |
CAS 57576-44-0
|
||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Cytarabine + GC-SF | Click to Show/Hide the Molecular Data of This Drug | |||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Experimental
Result(s) |
Increasing the dose of aclarubicin in CAG regimen seemed to be useful and relatively safe in relapsed and refractory AML. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II (TOP2) | Molecule Info | [3] | |

