Natural Product (NP) Details
| General Information of the NP (ID: NP8427) | |||||
|---|---|---|---|---|---|
| Name |
Phloroglucinol
|
||||
| Synonyms |
phloroglucinol; 108-73-6; Benzene-1,3,5-triol; 1,3,5-trihydroxybenzene; 1,3,5-benzenetriol; Phloroglucin; Phloroglucine; Spasfon-Lyoc; s-Trihydroxybenzene; 5-Hydroxyresorcinol; Benzene-s-triol; 3,5-Dihydroxyphenol; Phloroglucinol anhydrous; Benzene, trihydroxy; sym-Trihydroxybenzene; 5-Oxyresorcinol; Dilospan S; Floroglucinol; Floroglucin; 5-Oxyresorcinolphloroglucin; 1,3,5-THB; 1,3,5-Trihydroxycyclohexatriene; 1,3,5-Triol; MFCD00002286; Benzene, 1,3,5-trihydroxy-; UNII-DHD7FFG6YS; Phloroglucinol, anhydrous; 5-Benzenetriol; 1,2,5-trihydroxybenzene; NSC 1572; Phloroglucinol (JAN); DHD7FFG6YS; CHEMBL473159; CHEBI:16204; NSC-1572; Floroglucin [Czech]; Floroglucinol [Czech]; Phloroglucinol, 99+%, anhydrous; CCRIS 4147; NCGC00166270-01; EINECS 203-611-2; BRN 1341907; AI3-08848; phloroglucinol ts; 1,5-Benzenetriol; 13X; Dilospan S (TN); PubChem2603; Phloroglucinol [BAN]; 1,5-Trihydroxybenzene; 1,5-Triol; Benzene,3,5-trihydroxy-; WLN: QR CQ EQ; 1,3,5-trihydroxylbenzene; 1,3,5-trihydroxy benzene; 1,3,5-trihydroxy-benzene; 1,3, 5-Trihydroxybenzene; SCHEMBL26311; 1,5-Trihydroxycyclohexatriene; 4-06-00-07361 (Beilstein Handbook Reference); Phloroglucinol, p.a., 99%; DTXSID9048354; NSC1572; ZINC391883; ACT13446; ANW-15974; BBL018701; BDBM50249069; STL146346; phloroglucinol (1,3,5-benzenetriol); Phloroglucinol, >=99.0% (HPLC); AKOS000119851; AM84333; AS04650; DB12944; FS-4574; MCULE-5869131240; 1,3,5-Benzenetriol (ACD/Name 4.0); NCGC00166270-02; AC-10081; FT-0606511; FT-0673853; P0249; P1376; ST50214381; A-8565; C02183; D00152; A801919; Q899008; Q-200070; Phloroglucinol, plant cell culture tested, BioReagent; F0001-0177; Phloroglucinol, Used to detect the presence of wood fiber.; Phloroglucinol (anhydrous), European Pharmacopoeia (EP) Reference Standard
Click to Show/Hide
|
||||
| Species Origin | Dryopteris arguta ... | Click to Show/Hide | |||
| Dryopteris arguta | |||||
| Disease | Urinary system disease [ICD-11: GC2Z] | Approved | [1] | ||
| Structure |
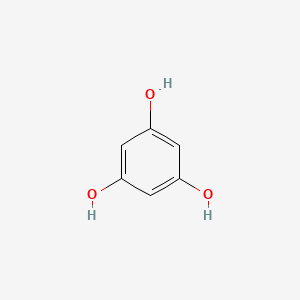
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-5.065
MDCK Permeability
-4.748
PAMPA
-
HIA
- - -
Distribution
VDss
-0.043
PPB
52.8%
BBB
- -
Metabolism
CYP1A2 inhibitor
+++
CYP1A2 substrate
+++
CYP2C19 inhibitor
++
CYP2C19 substrate
- - -
CYP2C9 inhibitor
++
CYP2C9 substrate
++
CYP2D6 inhibitor
+++
CYP2D6 substrate
+++
CYP3A4 inhibitor
+++
CYP3A4 substrate
- - -
CYP2B6 inhibitor
+++
CYP2B6 substrate
- - -
CYP2C8 inhibitor
+++
HLM Stability
+++
Excretion
CLplasma
11.771
T1/2
1.332
Toxicity
DILI
- - -
Rat Oral Acute Toxicity
-
FDAMDD
++
Respiratory
+
Human Hepatotoxicity
-
Ototoxicity
- -
Drug-induced Nephrotoxicity
- - -
Drug-induced Neurotoxicity
-
Hematotoxicity
- - -
Genotoxicity
+++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C6H6O3
|
||||
| PubChem CID | |||||
| Canonical SMILES |
C1=C(C=C(C=C1O)O)O
|
||||
| InChI |
1S/C6H6O3/c7-4-1-5(8)3-6(9)2-4/h1-3,7-9H
|
||||
| InChIKey |
QCDYQQDYXPDABM-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 108-73-6
|
||||
| ChEBI ID | |||||
| Herb ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Parecoxib | Gastrointestinal stromal tumor | Click to Show/Hide the Molecular Data of This Drug | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Experimental
Result(s) |
Parecoxib in combination with phloroglucinol for acute renal colic has a faster action, also reduces the demand of rescue analgesics. | |||||

