Drug Combination Details
| General Information of the Combination (ID: C28320) | |||||
|---|---|---|---|---|---|
| Name | Calcipotriol NP Info | + | Betamethasone dipropionate Drug Info | ||
| Structure |

|
+ |
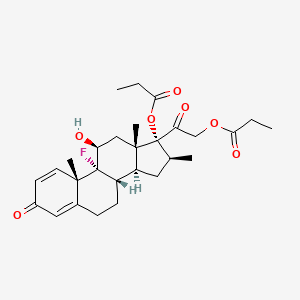
|
||
| Disease |
Plaque psoriasis
[ICD-11: EA90]
|
Investigative | [1] | ||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. Enhancing Drug Efficacy by This Combination | ||||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Experiment 1 Reporting the Effect of This Combination | [1] | |||||
| In-vivo Model | Clinical Trial | |||||
| Experimental
Result(s) |
The fixed combination treatment improves quality of life to a significantly greater extent than calcipotriol, with the once daily regimen most appreciated by patients, in both active disease and recurrency. | |||||
| References | ||||
|---|---|---|---|---|
| Reference 1 | Consensus on the use of the fixed combination calcipotriol/betamethasone dipropionate in the treatment of plaque psoriasis. G Ital Dermatol Venereol. 2012 Dec;147(6):609-24. | |||

