Drug Details
| General Information of the Drug (ID: DR2951) | ||||
|---|---|---|---|---|
| Name |
Betamethasone dipropionate
|
|||
| Synonyms |
BETAMETHASONE DIPROPIONATE; Diprolene; Diprosone; Diprolene AF; 5593-20-4; Alphatrex; Diproderm; Maxivate; Betamethasone 17,21-dipropionate; Diprosis; Psorion; Sch 11460; Betamethasone-17,21-dipropionate; Sch-11460; UNII-826Y60901U; S-3440; CHEBI:31276; beta-Methasone 17,21-dipropionate; 826Y60901U; Rinderon DP; Sernivo; (11-beta,16-beta)-9-Chloro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione; 9-fluoro-11beta-hydroxy-16beta-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl dipropanoate; (8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-17-(2-(propionyloxy)acetyl)-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl propionate.; [2-[(8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-17-propanoyloxy-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] propanoate; 2-((2S,10S,11S,13S,15S,17S,1R,14R)-1-fluoro-17-hydroxy-2,13,15-trimethyl-5-oxo -14-propanoyloxytetracyclo[8.7.0.0<2,7>.0<11,15>]heptadeca-3,6-dien-14-yl)-2-o xoethyl propanoate; 9-Fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione-17,21-dipropionate; EINECS 227-005-2; BRN 3638108; Betamethasone Dipropionate (Diprolene); S 3440; Diprolene (TN); NCGC00159360-02; NCGC00159443-02; Pregna-1,4-diene-3,20-dione, 9-fluoro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (11.beta.,16.beta.)-; Sernivo (TN); Rinderon-DP (TN); Betamethasone dipropionate [USAN:USP:JAN]; betamethasone-dipropionate; DSSTox_CID_2672; BETAMETHASONE DIPROP; SCHEMBL7519; 9-Fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate; DSSTox_RID_76683; DSSTox_GSID_22672; 9-Fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-di(propionate); CHEMBL1200384; DTXSID2022672; component of Betasone (Salt/Mix); component of Alphatrex (Salt/Mix); AMY22130; ZINC4212137; Tox21_113343; BDBM50421892; s1688; AKOS015969733; Betamethasone dipropionate (JP17/USP); CCG-269732; CS-7549; EBD2157850; KS-5303; NSC 758415; NCGC00159443-01; NCGC00159443-05; (11beta,16beta)-9-fluoro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl dipropanoate; HY-13571; Pregna-1,4-diene-3,20-dione, 9-fluoro-11-beta,17,21-trihydroxy-16-beta-methyl-, 17,21-dipropionate; Pregna-1,4-diene-3,20-dione, 9-fluoro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (11beta,16beta); ST024761; CAS-5593-20-4; B3166; D01637; AB01274713-01; AB01274713_02; Q4897349; BRD-K58148589-001-03-6; Betamethasone-17,21-dipropionate 100 microg/mL in Acetonitrile; (11.beta.,16.beta.)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate; 9-Fluoro-11.beta.,17,21-trihydroxy-16.beta.-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate; 9-Fluoro-11.beta.-hydroxy-16.beta.-methyl-3,20-dioxo-17-(propionyloxy)pregna-1,4-dien-21-yl propionate #; 9alpha-Fluoro-11beta,17alpha,21-trihydroxy-16beta-methyl-1,4-pregnadiene-3,20-dione 17,21-Dipropionate; 9alpha-fluoro-11beta,17alpha,21-trihydroxy-16beta-methyl-3,20-dioxopregna-1,4-diene- 17,21-diyl dipropionate; Pregna-1,4-diene-3,20-dione, 9-fluoro-11-.beta.,17,21-trihydroxy-16-.beta.-methyl-,17,21-dipropionate
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Plaque psoriasis [ICD-11: EA90] | Phase 3 | [1] | |
| Structure |
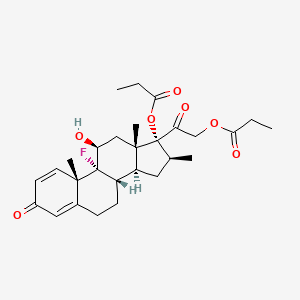
|
Click to Download Mol2D MOL |
||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C28H37FO7
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CCC(=O)OCC(=O)C1(C(CC2C1(CC(C3(C2CCC4=CC(=O)C=CC43C)F)O)C)C)OC(=O)CC
|
|||
| InChI |
1S/C28H37FO7/c1-6-23(33)35-15-22(32)28(36-24(34)7-2)16(3)12-20-19-9-8-17-13-18(30)10-11-25(17,4)27(19,29)21(31)14-26(20,28)5/h10-11,13,16,19-21,31H,6-9,12,14-15H2,1-5H3/t16-,19-,20-,21-,25-,26-,27-,28-/m0/s1
|
|||
| InChIKey |
CIWBQSYVNNPZIQ-XYWKZLDCSA-N
|
|||
| CAS Number |
CAS 5593-20-4
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Calcipotriol | Homo sapiens | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Clinical Trial | |||||
| Experimental
Result(s) |
The fixed combination treatment improves quality of life to a significantly greater extent than calcipotriol, with the once daily regimen most appreciated by patients, in both active disease and recurrency. | |||||

