Drug Combination Details
| General Information of the Combination (ID: C89029) | |||||
|---|---|---|---|---|---|
| Name | Selenium NP Info | + | Levothyroxine Drug Info | ||
| Structure |

|
+ |
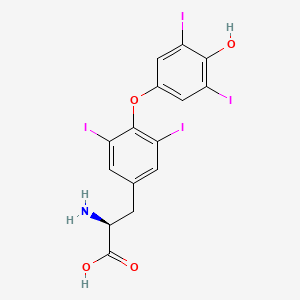
|
||
| Disease |
Chronic lymphocytic thyroiditis
[ICD-11: 5A03]
|
Investigative | [1] | ||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. Enhancing Drug Efficacy by This Combination | ||||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Experiment 1 Reporting the Effect of This Combination | [1] | |||||
| In-vivo Model | Clinical Trial | |||||
| Experimental
Result(s) |
Levothyroxine and selenium combination results in improved therapeutic effects than the levothyroxine monotherapy in preventing CLT progression. | |||||
| References | ||||
|---|---|---|---|---|
| Reference 1 | Levothyroxine monotherapy versus levothyroxine and selenium combination therapy in chronic lymphocytic thyroiditis. J Endocrinol Invest. 2017 Nov;40(11):1243-1250. | |||

