Drug Details
| General Information of the Drug (ID: DR8725) | ||||
|---|---|---|---|---|
| Name |
Levothyroxine
|
|||
| Synonyms |
L-thyroxine; levothyroxine; 51-48-9; thyroxine; thyroxin; Tetraiodothyronine; Thyrax; 3,3',5,5'-Tetraiodo-L-thyronine; synthroid; Thyratabs; Thyreoideum; Thyroxinal; Thyroxine iodine; Levothyroxin; (-)-Thyroxine; L-Thyroxin; 3,5,3',5'-Tetraiodo-L-thyronine; L-T4; Thyroxine (l); Levothyroxine sodium; DL-Thyroxin; O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-L-tyrosine; t4; Thyroxine (VAN); 3,5,3',5'-Tetraiodothyronine; Laevothyroxinum; T4 (Hormone); L-3,5,3',5'-Tetraiodothyronine; Levothyroxinum (acid); Laevothyroxinum (acid); Thryroxine, l-; Tetramet; Thyroxine I 125; Forthyron (TN); NSC 36397; Levothyroxinum; 3,3',5,5''-Tetraiodo-L-thyronine; CCRIS 6739; Henning; HSDB 3108; 3,5,3'5'-Tetraiodo-L-thyronine; UNII-Q51BO43MG4; Prestwick_548; L-Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-; 4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodo-L-phenylalanine; CHEBI:18332; O-(4-Hydroxy-3,5-diidophenyl)-3,5-diiodo-L-tyrosine; (S)-2-amino-3-(4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl)propanoic acid; 3-(4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl)alanine; beta-((3,5-Diiodo-4-hydroxyphenoxy)-3,5-diiodophenyl)alanine; 3-[4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]-L-alanine; CHEMBL1624; O-(4-Hydroxy-3,5-diiodophenyl)-3,5-diiodotyrosine; (2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]propanoic acid; C15H11I4NO4; Q51BO43MG4; 51-48-9 (free); THX; MFCD00002595; NCGC00164336-01; L-Thyroxine, 97+%; DSSTox_CID_3214; DSSTox_RID_76927; DSSTox_GSID_23214; Eutirox; Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-; LT4; SMR000059176; T44; Levothyroxine (BAN); EINECS 200-101-1; Levothyroxine [INN:BAN]; Synthroid (*Sodium salt*); BRN 2228515; SR-01000759430; NSC-36397; 2ceo; L-Tyrosine,; CAS-51-48-9; Spectrum_001076; 1hk1; 1hk2; 1hk3; 1hk4; 1hk5; 1y0x; CPD000059176; L-Thyroxine, free acid; SpecPlus_000871; Prestwick0_000403; Prestwick1_000403; Prestwick2_000403; Prestwick3_000403; Spectrum2_000573; Spectrum3_000611; Spectrum4_000128; Spectrum5_001500; Thyroxine, L- (8CI); bmse000923; Epitope ID:123889; T-3850; (2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodo-phenoxy)-3,5-diiodo-phenyl]propanoic acid; L-Thyroxine (T4) solution; BIDD:PXR0161; Levothroid (*Sodium salt*); SCHEMBL23098; BSPBio_000326; BSPBio_002142; KBioGR_000516; KBioSS_001556; MLS000028647; MLS002548901; DivK1c_006967; SPECTRUM1500774; SPBio_000386; SPBio_002265; BPBio1_000360; GTPL2635; DTXSID8023214; KBio1_001911; KBio2_001556; KBio2_004124; KBio2_006692; KBio3_001642; HMS1569A08; HMS1921I06; HMS2090P18; HMS2092K12; HMS2096A08; HMS2233J18; HMS3259M11; HMS3713A08; L-Thyroxine, >=98% (HPLC); Pharmakon1600-01500774; ZINC3830993; Tox21_112101; Tox21_302156; BDBM50301375; CCG-38738; NSC757434; s2599; AKOS015905129; Tox21_112101_1; AC-7504; AM83594; CS-1819; DB00451; NC00485; NSC-757434; SDCCGMLS-0066571.P001; 2-amino-3-[4-(4-hydroxy-3,5-diiodo-phenoxy)-3,5-diiodo-phenyl]-propanoic acid; 3,3'',5,5''-tetraiodo-L-thyronine; 3,5,3'',5''-tetraiodo-L-thyronine; NCGC00094852-03; NCGC00164336-02; NCGC00164336-03; NCGC00164336-04; NCGC00164336-05; NCGC00164336-06; NCGC00255368-01; AC-10465; HY-18341; SBI-0051608.P002; AB0010528; DB-006261; SW196731-3; C01829; D08125; J10324; O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-; AB00052176-08; AB00052176_09; AB00052176_10; 002T595; Q773449; SR-05000001567; SR-01000759430-2; SR-05000001567-1; BRD-K30685142-001-05-5; BRD-K30685142-001-08-9; Thyroxine (T4), IRMM(R) certified Reference Material; L-Thyroxine, powder, BioReagent, suitable for cell culture; UNII-QR0BV3BRIA component XUIIKFGFIJCVMT-LBPRGKRZSA-N; Levothyroxine, United States Pharmacopeia (USP) Reference Standard; Levothyroxine, Pharmaceutical Secondary Standard; Certified Reference Material; L-Thyroxine (T4) solution, 100 mug/mL in methanol with 0.1N NH3, ampule of 1 mL, certified reference material
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Hypo-thyroidism [ICD-11: 5A00] | Approved | [1] | |
| Structure |
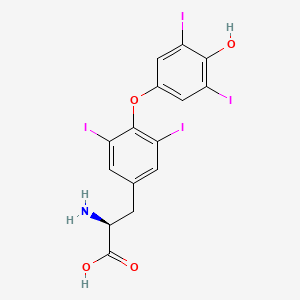
|
Click to Download Mol2D MOL |
||
| ADMET Property |
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 2: low solubility and high permeability
Elimination
Approximately 20% of T<sub>4 is eliminated in the stool
Half-life
The concentration or amount of drug in body reduced by one-half in 6 - 7 days (T4), and 1 - 2 days (T3)
Metabolism
The drug is metabolized via sequential deiodination
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 0.00644 micromolar/kg/day
Water Solubility
The ability of drug to dissolve in water is measured as 0.000585 mg/mL
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C15H11I4NO4
|
|||
| PubChem CID | ||||
| Canonical SMILES |
C1=C(C=C(C(=C1I)OC2=CC(=C(C(=C2)I)O)I)I)CC(C(=O)O)N
|
|||
| InChI |
1S/C15H11I4NO4/c16-8-4-7(5-9(17)13(8)21)24-14-10(18)1-6(2-11(14)19)3-12(20)15(22)23/h1-2,4-5,12,21H,3,20H2,(H,22,23)/t12-/m0/s1
|
|||
| InChIKey |
XUIIKFGFIJCVMT-LBPRGKRZSA-N
|
|||
| CAS Number |
CAS 51-48-9
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Selenium | Soil | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Clinical Trial | |||||
| Experimental
Result(s) |
Levothyroxine and selenium combination results in improved therapeutic effects than the levothyroxine monotherapy in preventing CLT progression. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Thyroid hormone receptor alpha (THRA) | Molecule Info | [3] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | Click to Show/Hide | ||
| 2 | Thyroid hormone signaling pathway | |||
| Pathway Interaction Database | RXR and RAR heterodimerization with other nuclear receptor | Click to Show/Hide | ||
| Reactome | Nuclear Receptor transcription pathway | Click to Show/Hide | ||
| WikiPathways | Endochondral Ossification | Click to Show/Hide | ||
| 2 | Nuclear Receptors | |||

