Drug Details
| General Information of the Drug (ID: DR4912) | ||||
|---|---|---|---|---|
| Name |
Memantine
|
|||
| Synonyms |
memantine; 19982-08-2; 3,5-dimethyladamantan-1-amine; Namenda; 1-Amino-3,5-dimethyladamantane; Ebixa; 3,5-Dimethyl-1-adamantanamine; Memantina; Memantinum [INN-Latin]; Memantina [INN-Spanish]; Memantinum; 1,3-Dimethyl-5-adamantanamine; Memantine [INN]; 3,5-Dimethyl-1-aminoadamantane; UNII-W8O17SJF3T; 3,5-Dimethyl-1-adamantylamine; 3,5-Dimethyltricyclo(3.3.1.1(3,7))decan-1-amine; Tricyclo[3.3.1.13,7]decan-1-amine, 3,5-dimethyl-; CHEMBL807; 3,5-dimethyladamantanylamine; W8O17SJF3T; D-145; CHEBI:64312; Memantine (INN); alzantin; NCGC00015705-04; DSSTox_CID_25174; DSSTox_RID_80723; DSSTox_GSID_45174; D 145; Memantine [INN:BAN]; CAS-19982-08-2; HSDB 7327; 3,5-dimethyltricyclo[3.3.1.1~3,7~]decan-1-amine; DMAA [Antiparkinson Agent]; Retyliumtosylate; 1,3-Dimethyl-5-aminoadamantane; DRG 0267; DRG-0267; Exiba (TN); Spectrum_000607; Prestwick0_000978; Prestwick1_000978; Prestwick2_000978; Prestwick3_000978; Spectrum2_001408; Spectrum3_000923; Spectrum4_001022; Spectrum5_001355; CBMicro_020348; Biomol-NT_000209; SCHEMBL2688; Lopac0_000861; Oprea1_480562; BSPBio_001015; KBioGR_001543; KBioSS_001087; DivK1c_000068; SPBio_001456; SPBio_002926; 1-amino-3,5-dimethyladamantan; BPBio1_001117; BPBio1_001270; GTPL4253; DTXSID5045174; SCHEMBL13213676; HMS500D10; KBio1_000068; KBio2_001087; KBio2_003655; KBio2_006223; KBio3_001926; 1-amino-3,5-dimethyl adamantane; 1-amino-3,5-dimethyl-adamantane; Tricyclo(3.3.1.1(3,7))decan-1-amine, 3,5-dimethyl-; NINDS_000068; (3,5-Dimethyl-1-adamantyl)amine; 3,5-Dimethyl-adamantan-1-ylamine; ALBB-013872; BCP29702; Tox21_110199; 3,5-Dimethyl-1-aminoadamantane HCl; ANW-56451; BBL000737; BDBM50062599; SBB002574; STK520682; AKOS000113995; AKOS015953276; AKOS016340748; Tox21_110199_1; CCG-204092; DB01043; DS-3152; MCULE-3726352671; NSC 757843; SDCCGSBI-0020417.P005; IDI1_000068; NCGC00015705-03; NCGC00015705-05; NCGC00015705-07; NCGC00015705-09; NCGC00015705-16; NCGC00024782-02; NCGC00024782-03; D145 pound>>D 145 pound>>D-145; ST057652; SBI-0020417.P004; AB0212255; AB00053600; BB 0216388; EU-0053634; FT-0649142; AZ0001-0553; C13736; D08174; S-1686; AB00053600-13; AB00053600_14; AB00053600_15; Q412189
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Alzheimer disease [ICD-11: 8A20] | Approved | [1] | |
| Structure |
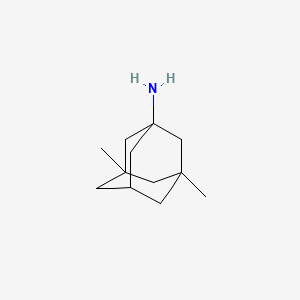
|
Click to Download Mol2D MOL |
||
| ADMET Property |
Absorption Tmax
The time to maximum plasma concentration (Tmax) is 3-7 h
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 3: high solubility and low permeability
Bioavailability
99% of drug becomes completely available to its intended biological destination(s)
Elimination
Approximately 48% of administered memantine is excreted unchanged in urine
Half-life
The concentration or amount of drug in body reduced by one-half in 60 - 100 hours
Metabolism
The drug is metabolized via the liver
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 1.324 micromolar/kg/day
Vd
The volume of distribution (Vd) of drug is 9-11 L/kg
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C12H21N
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CC12CC3CC(C1)(CC(C3)(C2)N)C
|
|||
| InChI |
1S/C12H21N/c1-10-3-9-4-11(2,6-10)8-12(13,5-9)7-10/h9H,3-8,13H2,1-2H3
|
|||
| InChIKey |
BUGYDGFZZOZRHP-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 19982-08-2
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Delta-9-tetrahydrocannabinol | Cannabis sativa | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Haloperidol-induced tremulous jaw movements (TJMs) in rats were used as a model of parkinsonian-like tremor. | |||||
| Experimental
Result(s) |
Memantine and THC supra-additively inhibit haloperidol-induced TJMs, suggesting that co-administration of these drugs might be a new approach to the treatment of tremor. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | N-methyl-D-aspartate receptor (NMDAR) | Molecule Info | [3] | |

