Drug Details
| General Information of the Drug (ID: DR5623) | ||||
|---|---|---|---|---|
| Name |
Ketamine
|
|||
| Synonyms |
ketamine; Ketaject; Ketalar; dl-Ketamine; CI 581 base; Special K; (+-)-Ketamine; Ketaminum; CLSTA 20; Ketanest; 6740-88-1; Ketalar base; 2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone; (+/-)-Ketamine; KETAMINE HCL; 2-(Methylamino)-2-(2-chlorophenyl)cyclohexanone; Ketolar; 2-(o-Chlorophenyl)-2-(methylamino)-cyclohexanone; 2-(2-chlorophenyl)-2-(methylamino)cyclohexan-1-one; NSC 70151; CHEMBL742; CHEBI:6121; Calypsol; 2-(2-Chloro-phenyl)-2-methylamino-cyclohexanone; 2-(o-Chlorophenyl)-2-(methylamino)cyclohexanone; Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-; NSC-70151; 100477-72-3; DEA No. 7285; NCGC00159480-02; NCGC00159480-03; Cetamina; Ketamine Base; Ketamine [INN:BAN]; DSSTox_CID_3187; Ketaminum [INN-Latin]; Cetamina [INN-Spanish]; DSSTox_RID_76912; Special K [street name]; DSSTox_GSID_23187; Ketoject; Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (+-)- (9CI); Ketamine (INN); PMI-150; CAS-6740-88-1; Tekam (TN); EINECS 229-804-1; (+/-)-2-(o-Chlorophenyl)-2-(methylamino)cyclohexanone; BRN 2216965; ketamina; HSDB 2180; Ketolar (Salt/Mix); Vetalar (Salt/Mix); Kalipsol (Salt/Mix); Ketanest (Salt/Mix); Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-, (+-)-; (.+/-.)-Ketamine; EC 229-804-1; Cyclohexanone, (.+-.)-; SCHEMBL16103; MLS001331674; DivK1c_000217; cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-; GTPL4233; (+/-)-2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone; DTXSID8023187; SCHEMBL17084881; KBio1_000217; NINDS_000217; HMS2272G05; NSC70151; Tox21_111703; Tox21_111704; BDBM50044140; DB01221; MCULE-4905246871; IDI1_000217; SMR000238141; C07525; D08098; Q243547; 2-(2-Chlorophenyl)-2-(methylamino)cyclohexanone #; J-505587; (.+/-.)-2-(O-Chlorophenyl)-2-(methylamino)cyclohexanone; 2-(2-Chloro-phenyl)-2-methylamino-cyclohexanone(Ketamine); Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)- (9CI); Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (+/-)-; Cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (.+/-.)-; Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-, (.+/-.)-; Cyclohexanone, 2-(o-chlorophenyl)-2-(methylamino)-, (+/-)- (8CI)
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Corneal disease [ICD-11: 9A78] | Approved | [1] | |
| Structure |
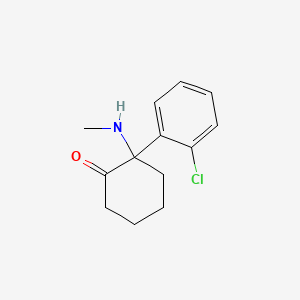
|
Click to Download Mol2D MOL |
||
| ADMET Property |
Absorption Cmax
The maximum plasma concentration (Cmax) of drug is 0.75 mg/L
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 1: high solubility and high permeability
Bioavailability
The bioavailability of drug is 93%
Clearance
The clearance of drug is 1.36 L/h/kg
Elimination
Pharmacokinetic studies have resulted in the recovery of 85-95% of the administered dose in urine mainly in the form of metabolites
Half-life
The concentration or amount of drug in body reduced by one-half in 186 minutes
Metabolism
The drug is metabolized via the hepatic
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 8.41285 micromolar/kg/day
Unbound Fraction
The unbound fraction of drug in plasma is 0.47%
Vd
The volume of distribution (Vd) of drug is 0.3713 L/kg
Water Solubility
The ability of drug to dissolve in water is measured as 200 mg/mL
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C13H16ClNO
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CNC1(CCCCC1=O)C2=CC=CC=C2Cl
|
|||
| InChI |
1S/C13H16ClNO/c1-15-13(9-5-4-8-12(13)16)10-6-2-3-7-11(10)14/h2-3,6-7,15H,4-5,8-9H2,1H3
|
|||
| InChIKey |
YQEZLKZALYSWHR-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 6740-88-1
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Magnesium | Magnesite | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | A total of one hundred and seventy-four Wistar rats (200-250 g) were used in the entire study. | |||||
| Experimental
Result(s) |
Fixed low dose of magnesium sulfate enhanced the temperature lowering effect of ketamine on baseline body temperature and morphine-induced hyperthermia. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | N-methyl-D-aspartate receptor (NMDAR) | Molecule Info | [3] | |

