Drug Details
| General Information of the Drug (ID: DR6918) | ||||
|---|---|---|---|---|
| Name |
Tacrine
|
|||
| Synonyms |
Tacrine; 1,2,3,4-tetrahydroacridin-9-amine; 321-64-2; Tetrahydroaminacrine; 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE; Tetrahydroaminoacridine; Cognex; Tetrahydroaminocrin; Tetrahydroaminocrine; 9-amino-1,2,3,4-tetrahydroacridine; 9-Acridinamine, 1,2,3,4-tetrahydro-; CS 12602; UNII-4VX7YNB537; 1,2,3,4-Tetrahydro-9-aminoacridine; Acridine, 1,2,3,4-tetrahydro-9-amino-; CHEMBL95; 5-Amino-6,7,8,9-tetrahydroacridine; 4VX7YNB537; CHEBI:45980; 5,6,7,8-tetrahydroacridin-9-amine; tacrine.HCl; NCGC00015054-06; Tacrina; Tacrinum; Tacrine [INN:BAN]; Tacrinum [INN-Latin]; Tacrina [INN-Spanish]; DSSTox_CID_17272; DSSTox_RID_79318; DSSTox_GSID_37272; 5,6,7,8-tetrahydroacridine-9-ylamine; Tacrinal (TN); CAS-321-64-2; Tacrine (INN); EINECS 206-291-2; 5-Amino-6,7,8,9-tetrahydroacridine (European); BRN 0147610; 1,2,3,4-Tetrahydro-acridin-9-ylamine; Tracine; 1acj; 2aow; 2aox; BBL001044; 1,2,3,4-Tetrahydro-9-acridineamine; Romotal (Salt/Mix); 9-Acridinamine,1,2,3,4-tetrahydro-; Spectrum_000416; Tocris-0965; 1mx1; Prestwick0_000329; Prestwick1_000329; Prestwick2_000329; Prestwick3_000329; Spectrum2_001812; Spectrum3_001709; Spectrum4_000819; Spectrum5_001402; Lopac-A-3773; cid_1935; SCHEMBL2828; 9-THA; NCIOpen2_003667; Lopac0_000036; Oprea1_008681; Acridine, 9-aminotetrahydro-; BSPBio_000337; BSPBio_003298; KBioGR_001337; KBioSS_000896; BIDD:GT0090; DivK1c_000936; SPBio_001823; SPBio_002258; BDBM8961; BPBio1_000371; GTPL6687; DTXSID1037272; KBio1_000936; KBio2_000896; KBio2_003464; KBio2_006032; KBio3_002518; NINDS_000936; HMS2089F19; HMS3743C11; BCP03564; Tox21_110073; Tox21_302277; ANW-46208; MFCD00046923; PDSP1_000330; PDSP2_000328; SBB003560; STK101308; ZINC19014866; AKOS000277493; Tox21_110073_1; BCP9000019; CCG-204132; DB00382; MCULE-3628803328; NE25597; SDCCGSBI-0050025.P004; VZ20338; 1,2,3,4-tetra -hydro-9-acridinamine; IDI1_000936; NCGC00015054-01; NCGC00015054-02; NCGC00015054-03; NCGC00015054-04; NCGC00015054-05; NCGC00015054-07; NCGC00015054-08; NCGC00015054-09; NCGC00015054-10; NCGC00015054-12; NCGC00024908-01; NCGC00024908-03; NCGC00024908-04; NCGC00024908-05; NCGC00255540-01; 1,2,3,4-tetrahydro-acridin-9-yl-amine; ST083243; Acridine, 9-amino-1,2,3,4-tetrahydro-; SBI-0050025.P003; AB0126098; DB-000650; 9-amino-1,2,3,4-tetrahydroacridine (THA); AB00053524; C-110; EU-0010966; FT-0631953; R9947; 9-Acridinamine, 1,2,3,4-tetrahydro- (9CI); A18628; C01453; D08555; AB00053524-17; AB00053524_18; AB00053524_19; AE-641/00604043; Q421076; 1,2,3,4-tetrahydroacridin-9-amine dihydrochloride; BRD-K81473089-003-03-0; BRD-K81473089-003-04-8; BRD-K81473089-003-15-4; SR-01000075593-13; Z56790573
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Alzheimer disease [ICD-11: 8A20] | Approved | [1] | |
| Structure |
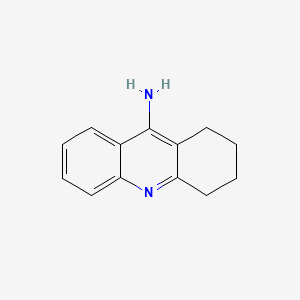
|
Click to Download Mol2D MOL |
||
| ADMET Property |
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 1: high solubility and high permeability
Bioavailability
The bioavailability of drug is 17%
Clearance
The drug present in the plasma can be removed from the body at the rate of 56 mL/min/kg
Elimination
0.5% of drug is excreted from urine in the unchanged form
Half-life
The concentration or amount of drug in body reduced by one-half in 2 - 4 hours
Metabolism
The drug is metabolized via the hepatic
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 11.5283 micromolar/kg/day
Unbound Fraction
The unbound fraction of drug in plasma is 0.25%
Vd
The volume of distribution (Vd) of drug is 349 +/- 193 L
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C13H14N2
|
|||
| PubChem CID | ||||
| Canonical SMILES |
C1CCC2=NC3=CC=CC=C3C(=C2C1)N
|
|||
| InChI |
1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15)
|
|||
| InChIKey |
YLJREFDVOIBQDA-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 321-64-2
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Decrease the Adverse Effect of This Drug | ||||||
| Schisandrol B | Schisandra chinensis | Click to Show/Hide the Molecular Data of This NP | ||||
| Decreasing Adverse Drug Reaction | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | In the Sch B pretreatment group in the hepatotoxicity study, mice (Adult male ICR mice) were treated intragastrically with Sch B at daily doses of 0.125 and 0.5 mmol/kg for 3 days. | |||||
| Experimental
Result(s) |
Sch B may be useful for reducing the potential hepatotoxicity of THA/bis(7)-THA in anti-Alzheimer's therapy. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Acetylcholinesterase (AChE) | Molecule Info | [3] | |
| KEGG Pathway | Glycerophospholipid metabolism | Click to Show/Hide | ||
| 2 | Cholinergic synapse | |||
| Panther Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | Click to Show/Hide | ||
| 2 | Muscarinic acetylcholine receptor 2 and 4 signaling pathway | |||
| 3 | Nicotinic acetylcholine receptor signaling pathway | |||
| Pathwhiz Pathway | Phospholipid Biosynthesis | Click to Show/Hide | ||
| Pathway Interaction Database | ATF-2 transcription factor network | Click to Show/Hide | ||
| WikiPathways | Monoamine Transport | Click to Show/Hide | ||
| 2 | Biogenic Amine Synthesis | |||
| 3 | Acetylcholine Synthesis | |||
| 4 | Integrated Pancreatic Cancer Pathway | |||

