Drug Combination Details
| General Information of the Combination (ID: C74903) | |||||
|---|---|---|---|---|---|
| Name | Schisandrol B NP Info | + | Tacrine Drug Info | ||
| Structure |

|
+ |
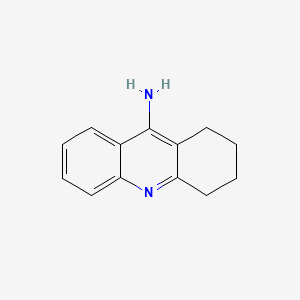
|
||
| Disease |
Alzheimer disease
[ICD-11: 8A20]
|
Investigative | [1] | ||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. Decreasing Adverse Drug Reaction by This Combination | ||||||
| Decreasing Adverse Drug Reaction | Click to Show/Hide | |||||
| Experiment 1 Reporting the Effect of This Combination | [1] | |||||
| In-vivo Model | In the Sch B pretreatment group in the hepatotoxicity study, mice (Adult male ICR mice) were treated intragastrically with Sch B at daily doses of 0.125 and 0.5 mmol/kg for 3 days. | |||||
| Experimental
Result(s) |
Sch B may be useful for reducing the potential hepatotoxicity of THA/bis(7)-THA in anti-Alzheimer's therapy. | |||||
| References | ||||
|---|---|---|---|---|
| Reference 1 | Schisandrin B protects against tacrine- and bis(7)-tacrine-induced hepatotoxicity and enhances cognitive function in mice. Planta Med. 2002 Mar;68(3):217-20. | |||

