Drug Details
| General Information of the Drug (ID: DR8320) | ||||
|---|---|---|---|---|
| Name |
Mitoxantrone
|
|||
| Synonyms |
DHAD; DHAQ; Dihydroxyanthraquinone; MIX; Misostol; Mitoxanthrone; Mitoxantron; Mitoxantrona; Mitoxantronum; Mitozantrone; DHAQ HCl; Mitoxantrone [INN]; Mitozantrone hydrochloride; Mitoxantrone 2HCl; Liposome Encapsulated Mitoxantrone (LEM); Misostol (TN); Mitoxantrona [INN-Spanish]; Mitoxantrone (INN); Mitoxantrone (free base); Mitoxantronum [INN-Latin]; Novantrone (TN); AN-584/42007670; Novantrone(R) (mitoxantrone for injection concentrate); DHAQ (*Diacetate salt*); MITOXANTRONE, Mitoxantrone Hydrochloride, Mitoxantrone dihydrochloride, MITOXANTHRONE HYDROCHLORIDE; MITOXANTRONE, 1,4-DIHYDROXY-5,8-BIS({2-[(2-HYDROXYETHYL)AMINO]ETHYL}AMINO)ANTHRA-9,10-QUINONE; 1,4-Bis(2-(2-hydroxyethylamino)ethyl)amino)-5,8-dihydroxyanthraquinone; 1,4-DIHYDROXY-5,8-BIS({2-[(2-HYDROXYETHYL)AMINO]ETHYL}AMINO)-9,10-ANTHRACENEDIONE; 1,4-Dihydroxy-5,8-bis(2-((2-hydroxyethyl)amino)ethylamino)-9,10-anthracenedione; 1,4-Dihydroxy-5,8-bis(5-hydroxy-3-azapentylamino)anthrachinon; 1,4-Dihydroxy-5,8-bis[2-(2-hydroxyethylamino)ethylamino]anthracene-9,10-dione; 1,4-Dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-9,10-anthracenedione; 1,4-dihydroxy-5,8-bis({2-[(2-hydroxyethyl)amino]ethyl}amino)anthra-9,10-quinone; 1,4-dihydroxy-5,8-bis({2-[(2-hydroxyethyl)amino]ethyl}amino)anthracene-9,10-dione; 5,8-Bis((2-((2-hydroxyethyl)amino)ethyl)amino)-1,4-dihydroxyanthraquinone; 9,10-Anthracenedione, 1,4-dihydroxy-5,8-bis((2-((2-hydroxyethyl)amino)ethyl)amino)-(9CI)
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | Approved | [1] | |
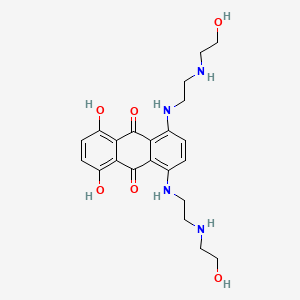
| Structure |
|
Click to Download Mol2D MOL |
||
| ADMET Property |
Absorption
The drug is poorly absorbed following oral administration
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 3: high solubility and low permeability
Clearance
The drug present in the plasma can be removed from the body at the rate of 7.9 mL/min/kg
Elimination
7% of drug is excreted from urine in the unchanged form
Half-life
The concentration or amount of drug in body reduced by one-half in 75 hours
Metabolism
The drug is metabolized via the hepatic
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 0.7784 micromolar/kg/day
Unbound Fraction
The unbound fraction of drug in plasma is 0.25%
Vd
Fluid volume that would be required to contain the amount of drug present in the body at the same concentration as in the plasma 12 L/kg
Water Solubility
The ability of drug to dissolve in water is measured as 7.5 mg/mL
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C22H28N4O6
|
|||
| PubChem CID | ||||
| Canonical SMILES |
C1=CC(=C2C(=C1NCCNCCO)C(=O)C3=C(C=CC(=C3C2=O)O)O)NCCNCCO
|
|||
| InChI |
1S/C22H28N4O6/c27-11-9-23-5-7-25-13-1-2-14(26-8-6-24-10-12-28)18-17(13)21(31)19-15(29)3-4-16(30)20(19)22(18)32/h1-4,23-30H,5-12H2
|
|||
| InChIKey |
KKZJGLLVHKMTCM-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 65271-80-9
|
|||
| ChEBI ID | ||||
| GDSC | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Silibinin | Carduus marianus | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vitro Model | DU145 | CVCL_0105 | Prostate carcinoma | Homo sapiens | ||
| LNCaP | CVCL_0395 | Prostate carcinoma | Homo sapiens | |||
| PC-3 | CVCL_0035 | Prostate carcinoma | Homo sapiens | |||
| Experimental
Result(s) |
The combination of silibinin and mitoxantrone exhibits a pattern of synergy in reducing cell viability with increased apoptosis. | |||||
| β. A List of Natural Product(s) Able to Decrease the Adverse Effect of This Drug | ||||||
| Reserpine | Rauvolfia serpentina | Click to Show/Hide the Molecular Data of This NP | ||||
| Decreasing Adverse Drug Reaction | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [3] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Molecule(s)
Regulation |
Up-regulation | Expression | CD80 | Molecule Info |
Pathway MAP
|
|
| Up-regulation | Expression | HLA-DRB5 | Molecule Info |
Pathway MAP
|
||
| Up-regulation | Expression | KLRK1 | Molecule Info |
Pathway MAP
|
||
| In-vitro Model | YAC-1 | CVCL_2244 | Mouse lymphoma | Mus musculus | ||
| B16-F10 | CVCL_0159 | Mouse melanoma | Mus musculus | |||
| In-vivo Model | For a xenograft model, Female C57BL/6 mice, 6-8 weeks of age, 18-20 g, were subcutaneously (s.c.) immunized in the abdominal region with 2x105, 5x105 or 1x106 B16F10 cells. | |||||
| Experimental
Result(s) |
The B16F10 tumor cell vaccine treated with MIP in combination with RP and VP was safe and efficient. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II (TOP2) | Molecule Info | [4] | |