Drug Details
| General Information of the Drug (ID: DR9473) | ||||
|---|---|---|---|---|
| Name |
Amantadine
|
|||
| Synonyms |
amantadine; 1-Adamantanamine; 768-94-5; adamantan-1-amine; 1-Adamantylamine; 1-Aminoadamantane; Adamantanamine; Adamantylamine; Aminoadamantane; Symmetrel; Amantidine; Symadine; Pk-merz; Adamantamine; 1-Adamantamine; Amantadina; Amantadinum; adamantan-1-ylamine; Gocovri; TCMDC-125869; 1-adamantaneamine; UNII-BF4C9Z1J53; 1-Aminotricyclo(3.3.1.1(sup 3,7))decane; NSC 341865; Tricyclo(3.3.1.13,7)decan-1-amine; tricyclo[3.3.1.1~3,7~]decan-1-amine; Tricyclo[3.3.1.1(3,7)]decan-1-amine; MFCD00074732; CHEMBL660; adamantanylamine; (3s,5s,7s)-Tricyclo[3.3.1.1~3,7~]decan-1-Amine; BF4C9Z1J53; CHEBI:2618; NSC341865; NSC-341865; 768-94-5 (FREE BASE); NCGC00015036-03; Tricyclo[3.3.1.13,7]decan-1-amine; Tricyclo[3.3.1.13,7]decane-1-amine; Amantadine Base; 1-Adamantanamine, 96%; DSSTox_CID_2117; Amantadine [INN:BAN]; DSSTox_RID_76493; Amantadinum [INN-Latin]; DSSTox_GSID_22117; Amantadina [INN-Spanish]; Amant; Amantadine (INN); CAS-768-94-5; NSC83653; 40933-03-7; HSDB 3202; Tricyclo(3.3.1.1(3,7))-decan-1-amine; Tricyclo(3.3.1.1(sup 3.7))decan-1-amine; EINECS 212-201-2; 1-Adamantanamine (8CI); ADAMANTANE,1-AMINO; adamantaneamine; BRN 2204333; adamantyl amine; AmantadineHCl; 1-admantaneamine; 1-Amantadine; Tricyclo(3.3.1.1(sup 3,7))decan-1-amine; 1-adamantanylamine; 1-adamantyl amine; Adamant-1-ylamine; 1-amino-adamantane; adamantane-1-amine; PubChem8733; Mantadine (Salt/Mix); Symmetrel (Salt/Mix); PubChem13903; Spectrum_000030; Tricyclo[3.3.1.1^3,7]decan-1-amine; Prestwick0_000407; Prestwick1_000407; Prestwick2_000407; Prestwick3_000407; Spectrum2_000081; Spectrum3_000291; Spectrum4_000134; Spectrum5_000772; tricyclo[3.3.1.1(3,7)]decan-1-ylamine; tricyclo[3.3.1.1(3,7)]decane-1-amine; 1-Adamantylamine, 97%; Lopac-A-1260; Tricyclo[3.3.1.1(sup3,7)]decan-1-amine; EC 212-201-2; SCHEMBL4098; NCIOpen2_001059; Lopac0_000004; Oprea1_248648; BSPBio_000334; BSPBio_001570; BSPBio_001822; KBioGR_000548; KBioSS_000390; BIDD:GT0757; DivK1c_000815; Exp-105-1 (Salt/Mix); SPBio_000002; SPBio_002273; BPBio1_000368; GTPL4128; SCHEMBL2619248; DTXSID8022117; SCHEMBL15672299; SCHEMBL20409394; SCHEMBL21309814; SCHEMBL21310017; KBio1_000815; KBio2_000390; KBio2_002958; KBio2_005526; KBio3_001322; NINDS_000815; (3R,5S,7s)-Adamantan-1-amine; 1-Adamantamine(1-Aminoadamantane); HMS1791O12; HMS1989O12; HMS3604O07; HMS3887I19; ZINC968256; ACT02873; ALBB-013871; BCP09869; STR04703; Tox21_110068; ANW-43040; BBL004977; BDBM50033369; HTS001826; s5499; SBB071506; STK298781; AKOS000113994; AKOS000119324; AKOS007930692; AKOS015935124; Tox21_110068_1; CCG-204100; CS-W008656; DB00915; MCULE-9686682307; SDCCGSBI-0049993.P005; IDI1_000815; NCGC00015036-01; NCGC00015036-02; NCGC00015036-04; NCGC00015036-05; NCGC00015036-06; NCGC00015036-07; NCGC00015036-08; NCGC00015036-09; NCGC00015036-11; NCGC00015036-13; NCGC00015036-24; NCGC00162039-01; NCGC00162039-02; NCGC00162039-03; NCGC00162039-04; NCGC00179597-01; AC-25879; ADS-5102, EXP 105-1; AK-77141; AS-14215; BP-13040; K333; ST097860; SBI-0049993.P004; AB0014168; WLN: L66 B6 A B- C 1B ITJ BZ; AB00514655; FT-0607338; C06818; D07441; M-4039; AB00053414-14; AB00053414-16; AB00053414_17; AB00053414_18; AB01275427-01; 768A945; A838887; AE-641/01634060; L000868; Q409761; J-650234; W-104338; BRD-K70330367-003-01-2; BRD-K70330367-003-03-8; Q27453436; F0001-1962
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Influenza [ICD-11: 1E30] | Approved | [1] | |
| Structure |
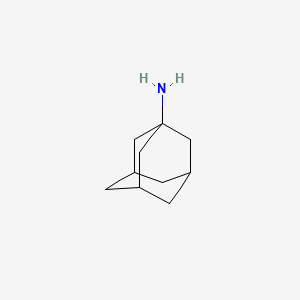
|
Click to Download Mol2D MOL |
||
| ADMET Property |
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 3: high solubility and low permeability
Bioavailability
90% of drug becomes completely available to its intended biological destination(s)
Clearance
The drug present in the plasma can be removed from the body at the rate of 4.8 mL/min/kg
Elimination
85% of drug is excreted from urine in the unchanged form
Half-life
The concentration or amount of drug in body reduced by one-half in 16 hours
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 37.78 micromolar/kg/day
Unbound Fraction
The unbound fraction of drug in plasma is 0.33%
Vd
Fluid volume that would be required to contain the amount of drug present in the body at the same concentration as in the plasma 6.6 L/kg
Water Solubility
The ability of drug to dissolve in water is measured as 50 mg/mL
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C10H17N
|
|||
| PubChem CID | ||||
| Canonical SMILES |
C1C2CC3CC1CC(C2)(C3)N
|
|||
| InChI |
1S/C10H17N/c11-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9H,1-6,11H2
|
|||
| InChIKey |
DKNWSYNQZKUICI-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 768-94-5
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| Ursodeoxycholic acid | Homo sapiens | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Molecule(s)
Regulation |
Up-regulation | Activity | STAT1 | Molecule Info |
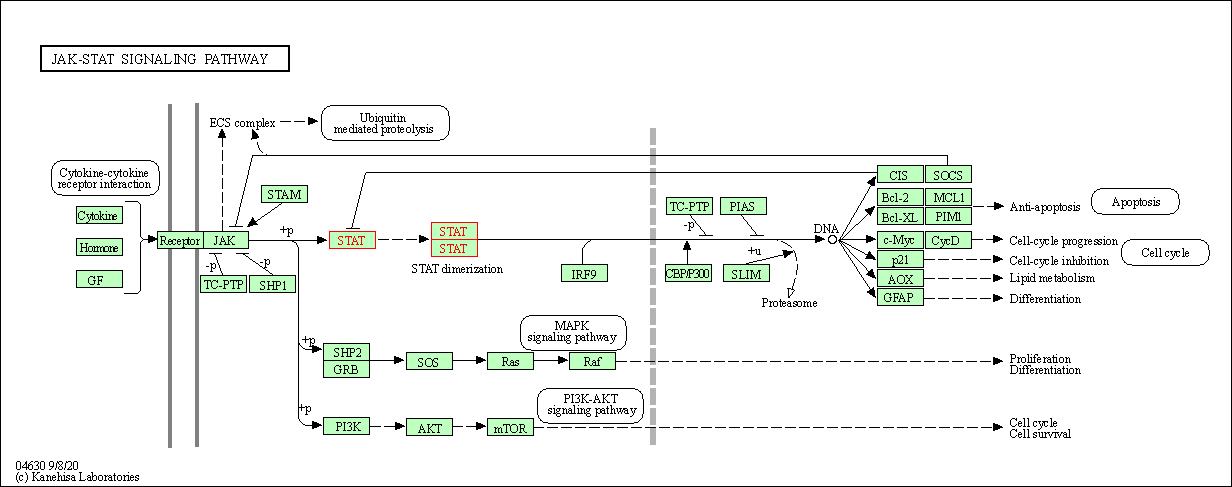
Pathway MAP
|
|
| In-vitro Model | Hep-G2 | CVCL_0027 | Hepatocellular carcinoma | Homo sapiens | ||
| Experimental
Result(s) |
The inhibition of viral gene replication was enhanced by the combination of triple combination of amantadine, ursodeoxycholic acidbip, and henyl dimethyl dicarboxylate. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Influenza M2 protein (Influ M) | Molecule Info | [3] | |
| WikiPathways | Influenza Life Cycle | Click to Show/Hide | ||