Natural Product (NP) Details
| General Information of the NP (ID: NP1003) | |||||
|---|---|---|---|---|---|
| Name |
Apalcillin
|
||||
| Synonyms |
Apalcillin; apalcilina; 63469-19-2; Apalcilline; Apalcillinum; UNII-3373RT9U7A; C25H23N5O6S; CHEBI:51691; 3373RT9U7A; PC-904; (2S,5R,6R)-6-{[(2R)-2-{[(4-hydroxy-1,5-naphthyridin-3-yl)carbonyl]amino}-2-phenylacetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 6beta-{(2R)-2-[(4-hydroxy-1,5-naphthyridin-3-yl)carboxamido]-2-phenylacetamido}-2,2-dimethylpenam-3alpha-carboxylic acid; Apalcillin [INN]; Apalcilina [INN-Spanish]; Apalcilline [INN-French]; Apalcillinum [INN-Latin]; BRN 6030446; SCHEMBL33854; CHEMBL3306902; GTPL10759; DTXSID601016509; PC904; ZINC59817121; (6R)-6-((R)-2-(4-Hydroxy-1,5-naphthyridin-3-carboxamido)-2-phenylacetamido)penicillansaeure; (2S,5R,6R)-3,3-dimethyl-7-oxo-6-[[(2R)-2-[(4-oxo-1H-1,5-naphthyridine-3-carbonyl)amino]-2-phenylacetyl]amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-((R)-2-(4-Hydroxy-1,5-naphthyridin-3-carboxamido)-2-phenylacetamido)-3,3-dimethyl-7-oxo-5-thia-1-azabicyclo(3.2.0)heptan-2-carbonsaeure; (2S,5R,6R)-6-((R)-2-(4-Hydroxy-1,5-naphthyridin-3-carboxamido)-2-phenylacetamido)-3,3-dimethyl-7-oxo-5-thia-1-azabicyclo(3.2.0)heptan-2-carboxylic acid; U326; WY-44417; WY-44,417; Q15633259; DIETHYL5,5-METHYLENEBIS(4-ETHYL-3-METHYL-2-PYRROLECARBOXYLATE); (2S,5R,6R)-6-(2-(4-hydroxy-1,5-naphthyridine-3-carboxamido)-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(((2R)-(((4-hydroxy-1,5-naphthyridin-3-yl)carbonyl)amino)phenylacetyl)amino)-3,3-dimethyl-7-oxo-, (2S,5R,6R)-
Click to Show/Hide
|
||||
| Species Origin | Escherichia coli ... | Click to Show/Hide | |||
| Escherichia coli | |||||
| Dauricine (Menispermum dauricum) | |||||
| Disease | Fungal infection [ICD-11: 1F29-1F2F] | Phase 2 | [1] | ||
| Gliomas of brain [ICD-11: 2A00.00] | Investigative | ||||
| Structure |
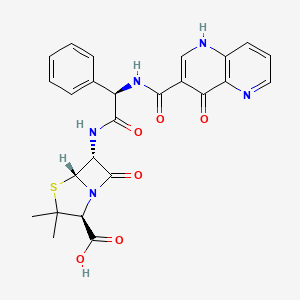
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-5.828
MDCK Permeability
-5.137
PAMPA
+++
HIA
++
Distribution
VDss
-0.688
PPB
72%
BBB
- - -
Metabolism
CYP1A2 inhibitor
- - -
CYP1A2 substrate
- - -
CYP2C19 inhibitor
- - -
CYP2C19 substrate
+++
CYP2C9 inhibitor
- - -
CYP2C9 substrate
- - -
CYP2D6 inhibitor
- - -
CYP2D6 substrate
- - -
CYP3A4 inhibitor
- -
CYP3A4 substrate
- - -
CYP2B6 inhibitor
- - -
CYP2B6 substrate
- - -
CYP2C8 inhibitor
- - -
HLM Stability
+++
Excretion
CLplasma
1.663
T1/2
1.187
Toxicity
DILI
+++
Rat Oral Acute Toxicity
- - -
FDAMDD
- - -
Respiratory
- - -
Human Hepatotoxicity
++
Ototoxicity
-
Drug-induced Nephrotoxicity
+++
Drug-induced Neurotoxicity
- - -
Hematotoxicity
+
Genotoxicity
+++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C25H23N5O6S
|
||||
| PubChem CID | |||||
| Canonical SMILES |
CC1(C(N2C(S1)C(C2=O)NC(=O)C(C3=CC=CC=C3)NC(=O)C4=CNC5=C(C4=O)N=CC=C5)C(=O)O)C
|
||||
| InChI |
1S/C25H23N5O6S/c1-25(2)19(24(35)36)30-22(34)17(23(30)37-25)29-21(33)15(12-7-4-3-5-8-12)28-20(32)13-11-27-14-9-6-10-26-16(14)18(13)31/h3-11,15,17,19,23H,1-2H3,(H,27,31)(H,28,32)(H,29,33)(H,35,36)/t15-,17-,19+,23-/m1/s1
|
||||
| InChIKey |
XMQVYNAURODYCQ-SLFBBCNNSA-N
|
||||
| CAS Number |
CAS 63469-19-2
|
||||
| ChEBI ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Gentamicin | Bacterial infection | Click to Show/Hide the Molecular Data of This Drug | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | For a xenograft model, mice were made neutropenic by intraperitoneal (ip) injection of cyclophosphamide at a dose of 100 mg/kg on days 0, 2,4. | |||||
| Experimental
Result(s) |
Apalcillin when combined with gentamicin is effective in treating serious P. aeruginosa bacteraemia in neutropenic mice. | |||||

