Natural Product (NP) Details
| General Information of the NP (ID: NP2704) | |||||
|---|---|---|---|---|---|
| Name |
Dopamine
|
||||
| Synonyms |
dopamine; 4-(2-Aminoethyl)benzene-1,2-diol; 3-Hydroxytyramine; 51-61-6; Dopamin; Hydroxytyramin; Oxytyramine; 3,4-dihydroxyphenethylamine; intropin; Dophamine; 2-(3,4-dihydroxyphenyl)ethylamine; 4-(2-Aminoethyl)catechol; hydroxytyramine; Dopaminum; 4-(2-Aminoethyl)pyrocatechol; 4-(2-Aminoethyl)-1,2-benzenediol; 3,4-Dihydroxyphenylethylamine; Dopamina; L-DOPAMINE; ASL 279; Pyrocatechol, 4-(2-aminoethyl)-; 1,2-Benzenediol, 4-(2-aminoethyl)-; Dopaminum [INN-Latin]; Dopamina [INN-Spanish]; 3-Hydroxtyramine; alpha-(3,4-Dihydroxyphenyl)-beta-aminoethane; KW-3-060; Sinemet; 4-(2-aminoethyl)-pyrocatechol; UNII-VTD58H1Z2X; Dynatra; NSC 173182; a-(3,4-Dihydroxyphenyl)-b-aminoethane; 4-(2-Aminoethyl)-1,2-bezenediol; VTD58H1Z2X; CHEBI:18243; NSC-173182; LDP; NCGC00015519-05; Pyrocatechol, 4-(2-aminoethyl)- (8CI); Dopamine [INN:BAN]; DSSTox_CID_2420; 1,2-Benzenediol, 4-(2-aminoethyl)- (9CI); DSSTox_RID_76584; DSSTox_GSID_22420; .alpha.-(3,4-Dihydroxyphenyl)-.beta.-aminoethane; (3H)-Dopamine; Pyrocatechol, 4-(2-aminoethyl)-, hydrochloride; 62-31-7 (HYDROCHLORIDE); CAS-51-61-6; Dopamine (INN); Medopa (TN); NSC169105; HSDB 3068; EINECS 200-110-0; Intropin [*hydrochloride*]; 4-(2-Aminoethyl)-1,2-benzenediol hydrochloride; SR-01000075366; .beta.-(3,4-Dihydroxyphenyl)ethylamine hydrochloride; m-Hydroxytyramine-; Dopamine (USAN)(*hydrochloride*); IP 498; Intropin (Salt/Mix); Spectrum_001012; 1,2-Benzenediol, 4-(2-aminoethyl)-, labeled with tritium; CHEMBL59; Spectrum2_001023; Spectrum3_000406; Spectrum4_000525; Spectrum5_000945; Lopac-H-8502; Biomol-NT_000001; bmse000909; bmse000933; SCHEMBL8505; 1, 4-(2-aminoethyl)-; Lopac0_000586; Oprea1_088821; BSPBio_001932; GTPL940; KBioGR_001129; KBioGR_002388; KBioGR_002484; KBioSS_001492; KBioSS_002393; KBioSS_002491; cid_65340; BIDD:ER0506; DivK1c_000780; SPECTRUM1505155; SPBio_001205; BPBio1_001123; 153C5321-5FEE-4B0B-8925-F388F0EEEBD1; DTXSID6022420; BDBM55121; KBio1_000780; KBio2_001492; KBio2_002388; KBio2_002484; KBio2_004060; KBio2_004956; KBio2_005052; KBio2_006628; KBio2_007524; KBio2_007620; KBio3_001152; KBio3_002867; KBio3_002962; ZINC33882; cMAP_000036; cMAP_000065; NINDS_000780; DOPAMINE, [7-3H(N)]; HMS3743I03; AMY40803; BCP34189; Tox21_110167; 2-(3, 4-Dihydroxyphenyl)ethylamine; 2-(3,4-dihydroxyphenyl) ethylamine; ANW-54385; BBL013043; MFCD00130258; NSC173182; SBB004044; STK301601; 3,4-DihydroxyphenylA currencythylamin; AKOS003790978; Tox21_110167_1; CCG-204675; DB00988; FS-5341; MCULE-7558764100; SDCCGSBI-0050568.P005; 2-(3,4-Dihydroxyphenyl)-1-ethanamine; 4-(2-Amino-ethyl)-benzene-1,2-diol; 4-(2-Aminoethyl)-1,2-benzenediol #; IDI1_000780; UPCMLD0ENAT5885989:001; NCGC00015519-01; NCGC00015519-02; NCGC00015519-03; NCGC00015519-04; NCGC00015519-07; NCGC00015519-08; NCGC00015519-09; NCGC00015519-10; NCGC00015519-11; NCGC00015519-25; NCGC00096050-01; NCGC00096050-02; NCGC00096050-03; NCGC00096050-04; NCGC00096050-05; 50444-17-2; AK100905; BP-23276; ST048774; 4-(2-aminoethyl)pyrocatechol;hydrochloride; SBI-0050568.P004; FT-0698513; T7923; 2-(4-Hydroxy-5-oxylatophenyl)-1-ethanaminium; C03758; D07870; 13510-EP2269989A1; 13510-EP2270011A1; 13510-EP2272537A2; 13510-EP2272825A2; 13510-EP2272847A1; 13510-EP2275420A1; 13510-EP2277882A1; 13510-EP2280010A2; 13510-EP2281559A1; 13510-EP2281815A1; 13510-EP2281819A1; 13510-EP2284169A1; 13510-EP2284170A1; 13510-EP2284171A1; 13510-EP2286811A1; 13510-EP2287161A1; 13510-EP2287162A1; 13510-EP2287165A2; 13510-EP2287166A2; 13510-EP2292620A2; 13510-EP2295437A1; 13510-EP2295439A1; 13510-EP2298312A1; 13510-EP2298313A1; 13510-EP2298731A1; 13510-EP2298734A2; 13510-EP2298758A1; 13510-EP2298759A1; 13510-EP2298775A1; 13510-EP2298776A1; 13510-EP2301540A1; 13510-EP2301933A1; 13510-EP2305260A1; 13510-EP2305633A1; 13510-EP2305640A2; 13510-EP2305644A1; 13510-EP2305648A1; 13510-EP2305650A1; 13510-EP2305652A2; 13510-EP2305656A1; 13510-EP2305659A1; 13510-EP2305664A1; 13510-EP2305675A1; 13510-EP2305689A1; 13510-EP2308828A2; 13510-EP2308867A2; 13510-EP2308870A2; 13510-EP2308875A1; 13510-EP2311494A1; 13510-EP2311801A1; 13510-EP2311802A1; 13510-EP2311803A1; 13510-EP2311818A1; 13510-EP2311827A1; 13510-EP2311828A1; 13510-EP2311835A1; 13510-EP2314571A2; 13510-EP2316470A2; 13510-EP2316836A1; 13510-EP2371814A1; 4-(2-azanylethyl)benzene-1,2-diol;hydrochloride; AB00053463-12; AB00053463_13; AB00053463_14; L000232; Q170304; SR-01000075366-7; Oseltamivir-d3;Hydroxytyramin;4-(2-Aminoethyl)benzene-1,2-diol; 70097-41-5
Click to Show/Hide
|
||||
| Species Origin | Lactiplantibacillus plantarum ... | Click to Show/Hide | |||
| Lactiplantibacillus plantarum | |||||
| Disease | Parkinson's disease [ICD-11: 8A00] | Approved | [1] | ||
| Structure |
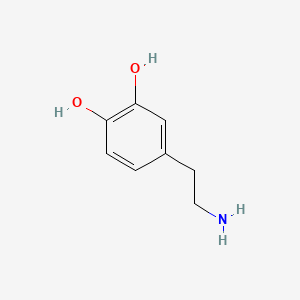
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-5.025
MDCK Permeability
-4.717
PAMPA
++
HIA
++
Distribution
VDss
-0.122
PPB
4.7%
BBB
- - -
Metabolism
CYP1A2 inhibitor
- - -
CYP1A2 substrate
+++
CYP2C19 inhibitor
- - -
CYP2C19 substrate
+++
CYP2C9 inhibitor
- - -
CYP2C9 substrate
+++
CYP2D6 inhibitor
- - -
CYP2D6 substrate
+++
CYP3A4 inhibitor
- - -
CYP3A4 substrate
- - -
CYP2B6 inhibitor
- - -
CYP2B6 substrate
- - -
CYP2C8 inhibitor
- - -
HLM Stability
- - -
Excretion
CLplasma
16.503
T1/2
2.121
Toxicity
DILI
- - -
Rat Oral Acute Toxicity
-
FDAMDD
+
Respiratory
+
Human Hepatotoxicity
-
Ototoxicity
-
Drug-induced Nephrotoxicity
- - -
Drug-induced Neurotoxicity
- -
Hematotoxicity
- - -
Genotoxicity
- -
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C8H11NO2
|
||||
| PubChem CID | |||||
| Canonical SMILES |
C1=CC(=C(C=C1CCN)O)O
|
||||
| InChI |
1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2
|
||||
| InChIKey |
VYFYYTLLBUKUHU-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 51-61-6
|
||||
| ChEBI ID | |||||
| Herb ID | |||||
| SymMap ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Lidocaine | Corneal disease | Click to Show/Hide the Molecular Data of This Drug | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Male adult Sprague-Dawley rats, weighing 201 to 251 g were used in this study. | |||||
| Experimental
Result(s) |
Serotonin and dopamine produce dose-related cutaneous analgesic effects as an infiltrative anesthetic. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Dopamine D2 receptor (D2R) | Molecule Info | [3] | |
| KEGG Pathway | Rap1 signaling pathway | Click to Show/Hide | ||
| 2 | cAMP signaling pathway | |||
| 3 | Neuroactive ligand-receptor interaction | |||
| 4 | Gap junction | |||
| 5 | Dopaminergic synapse | |||
| 6 | Parkinson's disease | |||
| 7 | Cocaine addiction | |||
| 8 | Alcoholism | |||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | Click to Show/Hide | ||
| 2 | Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | |||
| 3 | Dopamine receptor mediated signaling pathway | |||
| 4 | Nicotine pharmacodynamics pathway | |||
| Reactome | Dopamine receptors | Click to Show/Hide | ||
| 2 | G alpha (i) signalling events | |||
| WikiPathways | Hypothetical Network for Drug Addiction | Click to Show/Hide | ||
| 2 | Monoamine GPCRs | |||
| 3 | GPCRs, Class A Rhodopsin-like | |||
| 4 | Genes and (Common) Pathways Underlying Drug Addiction | |||
| 5 | GPCR ligand binding | |||
| 6 | GPCR downstream signaling | |||
| 7 | Nicotine Activity on Dopaminergic Neurons | |||

