Natural Product (NP) Details
| General Information of the NP (ID: NP5811) | |||||
|---|---|---|---|---|---|
| Name |
Tetrodotoxin
|
||||
| Synonyms |
TETRODOTOXIN; 4368-28-9; Tarichatoxin; Maculotoxin; Spheroidine; Tetrodontoxin; Fugu poison; CHEMBL507974; UNII-3KUM2721U9; Tetrodotoxine; Tetrodoxin; TTX; (1R,5R,6R,7R,9S,11S,12S,13S,14S)-3-amino-14-(hydroxymethyl)-8,10-dioxa-2,4-diazatetracyclo[7.3.1.1~7,11~.0~1,6~]tetradec-3-ene-5,9,12,13,14-pentol (non-preferred name); Babylonia japonica toxin 1; tettrodotoxin; BJT 1; 3KUM2721U9; CCRIS 9328; HSDB 3543; 9SR; Octahydro-12-(hydroxymethyl)-2-imino-5,9:7,10a-dimethano-10aH-(1,3)dioxocino(6,5-d)pyrimidine-4,7,10,11,12-pentol; EINECS 224-458-8; BRN 0049176; 4-27-00-08206 (Beilstein Handbook Reference); SCHEMBL6406675; 5,9:7,10a-Dimethano-10aH-(1,3)dioxocino(6,5-d)pyrimidine-4,7,10,11,12-pentol, octahydro-12-(hydroxymethyl)-2-imino-, (4R,4aR,5R,7S,9S,10S,10aR,11S,12S)-; BDBM50344821; ZINC13780673; (4R-(4alpha,4aalpha,5alpha,7alpha,9alpha,10alpha,10abeta,11S*,12S*))-Octahydro-12-(hydroxymethyl)-2-imino-5,9:7,10a-dimethano-10aH-(1,3)dioxocino(6,5-d) pyrimidine-4,7,10,11,12-pentol; 5,9:7,10a-Dimethano-10ah-(1,3)dioxocino(6,5-d)pyrimidine-4,7,10,11,12-pentol, octahydro-12-(hydroxymethyl)-2-imino-, (4R-(4alpha,4aalpha,5alpha,7alpha,9alpha,10alpha,10abeta,11S*,12S*))-; X5934; Q-100286; (1R,5R,6R,7R,9S,11S,12S,13S,14S)-3-amino-14-(hydroxymethyl)-8,10-dioxa-2,4-diazatetracyclo[7.3.1.17,11.01,6]tetradec-3-ene-5,9,12,13,14-pentol; 10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diazatetracyclo[7.3.1.13,11.03,8]tetradecane-1,2,7,10,14-pentaol; 10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diazatetracyclo[7.3.1.13,11.03,8]tetradecane-1,2,7,10,14-pentaolCitrate; 5,9:7,10a-Dimethano-10aH-(1,3)dioxocino(6,5-d)pyrimidine-4,7,10,11,12-pentol, octahydro-12-(hydroxymethyl)-2-imino-
Click to Show/Hide
|
||||
| Species Origin | Takifugu rubripes ... | Click to Show/Hide | |||
| Takifugu rubripes | |||||
| Disease | Bacterial infection [ICD-11: 1A00-1C4Z] | Approved | [1] | ||
| Structure |
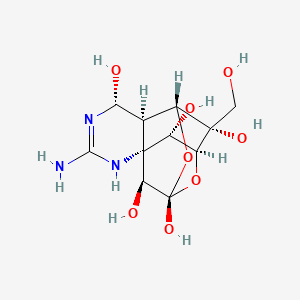
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-5.722
MDCK Permeability
-4.845
PAMPA
+++
HIA
+++
Distribution
VDss
-0.348
PPB
12.8%
BBB
- - -
Metabolism
CYP1A2 inhibitor
- - -
CYP1A2 substrate
- - -
CYP2C19 inhibitor
- - -
CYP2C19 substrate
- - -
CYP2C9 inhibitor
- - -
CYP2C9 substrate
- - -
CYP2D6 inhibitor
- - -
CYP2D6 substrate
- - -
CYP3A4 inhibitor
- - -
CYP3A4 substrate
- - -
CYP2B6 inhibitor
- - -
CYP2B6 substrate
- - -
CYP2C8 inhibitor
-
HLM Stability
- - -
Excretion
CLplasma
1.387
T1/2
2.439
Toxicity
DILI
-
Rat Oral Acute Toxicity
++
FDAMDD
+
Respiratory
+++
Human Hepatotoxicity
-
Ototoxicity
++
Drug-induced Nephrotoxicity
++
Drug-induced Neurotoxicity
- -
Hematotoxicity
-
Genotoxicity
+++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C11H17N3O8
|
||||
| PubChem CID | |||||
| Canonical SMILES |
C(C1(C2C3C(N=C(NC34C(C1OC(C4O)(O2)O)O)N)O)O)O
|
||||
| InChI |
1S/C11H17N3O8/c12-8-13-6(17)2-4-9(19,1-15)5-3(16)10(2,14-8)7(18)11(20,21-4)22-5/h2-7,15-20H,1H2,(H3,12,13,14)/t2-,3-,4-,5+,6-,7+,9+,10-,11+/m1/s1
|
||||
| InChIKey |
CFMYXEVWODSLAX-QOZOJKKESA-N
|
||||
| CAS Number |
CAS 4368-28-9
|
||||
| Herb ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Lidocaine | Corneal disease | Click to Show/Hide the Molecular Data of This Drug | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vitro Model | Chinese hamster ovary cell line | Healthy | Cricetulus griseus | |||
| In-vivo Model | The animal models were established in Sprague Dawley rats by continuous infusion of aconitine into the caudal vein or vena jugularis externa. | |||||
| Experimental
Result(s) |
The anti-arrhythmic effect of the combination of TTX and LID was greater than that of either TTX or LID alone. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Sodium channel alpha Nav1.5 (SCN5A) | Molecule Info | [3] | |
| KEGG Pathway | Adrenergic signaling in cardiomyocytes | Click to Show/Hide | ||
| Pathwhiz Pathway | Muscle/Heart Contraction | Click to Show/Hide | ||
| Reactome | Interaction between L1 and Ankyrins | Click to Show/Hide | ||
| WikiPathways | SIDS Susceptibility Pathways | Click to Show/Hide | ||
| 2 | Cardiac Progenitor Differentiation | |||

