Natural Product (NP) Details
| General Information of the NP (ID: NP7145) | |||||
|---|---|---|---|---|---|
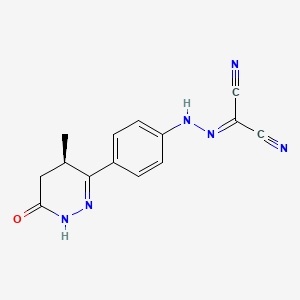
| Name |
Levosimendan
|
||||
| Synonyms |
LEVOSIMENDAN; 141505-33-1; Simdax; (-)-OR-1259; UNII-C6T4514L4E; OR-1259; CHEBI:50567; C6T4514L4E; (R)-N-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)carbonohydrazonoyl dicyanide; Levosimedan; DSSTox_CID_26445; DSSTox_RID_81620; DSSTox_GSID_46445; (R)-Simendan; levosimendanum; SMR002529692; Simdax (TN); CAS-141505-33-1; Levosimendan (USAN/INN); Levosimendan [USAN:INN]; OR 1259; OR1259; ((4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazono)propanedinitrile; (R)-((4-(1,4,5,6-Tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazono) propanedintrile; Mesoxalonitrile (-)-(p((R)-1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazone; SCHEMBL83243; MLS003899227; MLS006010741; CHEMBL2051955; DTXSID9046445; Levosimendan, >=98% (HPLC); HMS3264G03; HMS3884N17; KUC109648N; Pharmakon1600-01502356; ACT02710; BCP07048; ZINC3915645; Tox21_112191; Tox21_113768; BDBM50469700; MFCD00867135; NSC759644; s2446; AKOS015895214; Tox21_112191_1; AC-1752; AM84381; CCG-213048; DB00922; DS-8918; NSC 759644; NSC-759644; SB17415; ({4-[(4R)-4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl]phenyl}hydrazono)propanedintrile; KSC-210-010; Mesoxalonitrile (p-((R)-1,4,5,6-tetrahydro-4-methyl-6-oxo-pyridazinyl)phenyl)hydrazone; NCGC00253641-01; NCGC00263564-01; NCGC00263564-02; HY-14286; SW219172-1; A11874; D04720; AB01562970_01; AB01562970_02; 741L087; A807767; Q162541; SR-01000931342; SR-01000931342-2; 1-beta-D-Ribofuranose-1H-1,2,4-triazole-3-methylcarbonate; UNII-349552KRHK component WHXMKTBCFHIYNQ-SECBINFHSA-N; (R)-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)carbonohydrazonoyl dicyanide; 1-cyano-N-{4-[(4R)-4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl]phenyl}methanecarbohydrazonoyl cyanide; 2-[[4-[(4R)-4-methyl-6-oxidanylidene-4,5-dihydro-1H-pyridazin-3-yl]phenyl]hydrazinylidene]propanedinitrile
Click to Show/Hide
|
||||
| Species Origin | Homo sapiens ... | Click to Show/Hide | |||
| Homo sapiens | |||||
| Disease | Heart failure [ICD-11: BD10] | Approved | [1] | ||
| Structure |
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-4.823
MDCK Permeability
-4.871
PAMPA
++
HIA
- - -
Distribution
VDss
-0.692
PPB
97.7%
BBB
- - -
Metabolism
CYP1A2 inhibitor
++
CYP1A2 substrate
- - -
CYP2C19 inhibitor
+++
CYP2C19 substrate
- - -
CYP2C9 inhibitor
+++
CYP2C9 substrate
- - -
CYP2D6 inhibitor
- - -
CYP2D6 substrate
- - -
CYP3A4 inhibitor
-
CYP3A4 substrate
+++
CYP2B6 inhibitor
- - -
CYP2B6 substrate
- - -
CYP2C8 inhibitor
+++
HLM Stability
- - -
Excretion
CLplasma
3.078
T1/2
1.093
Toxicity
DILI
+++
Rat Oral Acute Toxicity
++
FDAMDD
+++
Respiratory
+++
Human Hepatotoxicity
-
Ototoxicity
+
Drug-induced Nephrotoxicity
- - -
Drug-induced Neurotoxicity
+++
Hematotoxicity
-
Genotoxicity
+++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C14H12N6O
|
||||
| PubChem CID | |||||
| Canonical SMILES |
CC1CC(=O)NN=C1C2=CC=C(C=C2)NN=C(C#N)C#N
|
||||
| InChI |
1S/C14H12N6O/c1-9-6-13(21)19-20-14(9)10-2-4-11(5-3-10)17-18-12(7-15)8-16/h2-5,9,17H,6H2,1H3,(H,19,21)/t9-/m1/s1
|
||||
| InChIKey |
WHXMKTBCFHIYNQ-SECBINFHSA-N
|
||||
| CAS Number |
CAS 141505-33-1
|
||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Adverse Effect can be Decreased by This NP | ||||||
| Esmolol | Supraventricular tachyarrhythmia | Click to Show/Hide the Molecular Data of This Drug | ||||
| Decreasing Adverse Drug Reaction | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | A case report | |||||
| Experimental
Result(s) |
levosimendan in combination with a beta-adrenergic antagonist may have beneficial effects in patients with cardiogenic shock who exhibit tachycardia in response to inotropic agents. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Troponin C (TNNC1) | Molecule Info | [3] | |
| KEGG Pathway | Calcium signaling pathway | Click to Show/Hide | ||
| 2 | Cardiac muscle contraction | |||
| 3 | Adrenergic signaling in cardiomyocytes | |||
| 4 | Hypertrophic cardiomyopathy (HCM) | |||
| 5 | Dilated cardiomyopathy | |||
| Pathwhiz Pathway | Muscle/Heart Contraction | Click to Show/Hide | ||
| Reactome | Striated Muscle Contraction | Click to Show/Hide | ||
| WikiPathways | Striated Muscle Contraction | Click to Show/Hide | ||
| 2 | Muscle contraction | |||

