Natural Product (NP) Details
| General Information of the NP (ID: NP8392) | |||||
|---|---|---|---|---|---|
| Name |
Pseudoephedrine
|
||||
| Synonyms |
PSEUDOEPHEDRINE; (+)-Pseudoephedrine; d-Pseudoephedrine; Isoephedrine; Sudafed; trans-Ephedrine; d-Isoephedrine; Psi-ephedrin; Psi-ephedrine; d-psi-Ephedrine; L(+)-psi-Ephedrine; (+)-threo-Ephedrine; L-(+)-Pseudoephedrine; (+)-psi-Ephedrine; 90-82-4; Pseudoefedrina; Pseudoephedrinum; Besan; (1S,2S)-2-(methylamino)-1-phenylpropan-1-ol; (+)-(1S,2S)-Pseudoephedrine; Pseudoephedrine d-form; (1S,2S)-Pseudoephedrine; (1S,2S)-(+)-Pseudoephedrine; d-psi-2-Methylamino-1-phenyl-1-propanol; UNII-7CUC9DDI9F; 7CUC9DDI9F; 90-82-4 (free base); Pseudoephedrine, (+)-; CHEBI:51209; Pseudoephedrine, L-(+)-; alpha-(1-(Methylamino)ethyl)benzyl alcohol; Pseudoephedrine (D); Pseudoefedrina [INN-Spanish]; Pseudoephedrinum [INN-Latin]; (+) threo-2-(methylamino)-1-phenyl-1-propanol; Pseudoephedrine [INN:BAN]; Pseudoephedrine Ephedrine; psi-Ephedrine, (+)-; 2-(Methylamino)-1-phenyl-1-propanol; Neodurasina (TN); Acunaso (TN); pseudophedrine sulphate; HSDB 3177; Pseudoephedrine (INN); Pseudoephedrine polistirex; EINECS 202-018-6; Benzenemethanol, .alpha.-[(1S)-1-(methylamino)ethyl]-, (.alpha.S)-; (I)-Ephedrin; ephedrine-(racemic); (+) pseudoephedrine; d-Pseudoephedrine base; Spectrum_000878; Benzenemethanol, alpha-(1-(methylamino)ethyl)-, (S-(R*,R*))-; Spectrum2_001303; Spectrum3_001771; Spectrum4_001162; Spectrum5_000650; Lopac-E-3250; (1S,2S) pseudoephedrine; (1S,2S)-2-methylamino-1-phenylpropan-1-ol; (1s, 2s) pseudoephedrine; EC 202-018-6; SCHEMBL4368; CHEMBL1590; (1S,2S)-2-(methylamino)-1-phenyl-propan-1-ol; BSPBio_003261; KBioGR_001763; KBioSS_001358; BIDD:GT0817; DivK1c_000451; SPBio_001365; GTPL7286; DTXSID0023537; KBio1_000451; KBio2_001358; KBio2_003926; KBio2_006494; KBio3_002762; ZINC20259; (1s, 2s)-(+)-pseudoephedrine; NINDS_000451; Benzenemethanol, alpha-((1S)-1-(methylamino)ethyl)-, (alphaS)-; Benzenemethanol, alpha-((1S)-1-(methylamino)ethyl)-, (alpha-S)-; PDSP1_001347; AKOS025401512; DB00852; MCULE-5756045923; IDI1_000451; (1S,2S)-(+)-Pseudoephedrine, 98%; NCGC00015408-01; NCGC00178180-01; NCI60_002955; SBI-0051498.P003; C02765; D08449; 064P256; Q263958; BRD-K84175871-003-02-2; Benzenemethanol, alpha-((1S)-1-(methylamino)ethyl)-, (alpha-S)- (9CI); (S,S)-(+)-Pseudoephedrine solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material
Click to Show/Hide
|
||||
| Species Origin | Ephedra altissima ... | Click to Show/Hide | |||
| Ephedra altissima | |||||
| Disease | Breathing abnormality [ICD-11: MD11] | Approved | [1] | ||
| Structure |
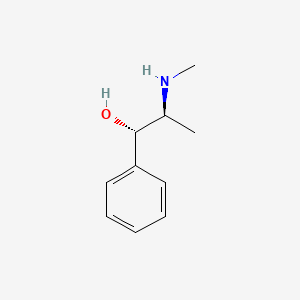
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-5.008
MDCK Permeability
-4.754
PAMPA
+++
HIA
- - -
Distribution
VDss
0.495
PPB
28.5%
BBB
- - -
Metabolism
CYP1A2 inhibitor
+++
CYP1A2 substrate
+++
CYP2C19 inhibitor
- - -
CYP2C19 substrate
+++
CYP2C9 inhibitor
- - -
CYP2C9 substrate
- - -
CYP2D6 inhibitor
+++
CYP2D6 substrate
+++
CYP3A4 inhibitor
- - -
CYP3A4 substrate
- -
CYP2B6 inhibitor
- - -
CYP2B6 substrate
- - -
CYP2C8 inhibitor
- - -
HLM Stability
-
Excretion
CLplasma
5.138
T1/2
4.412
Toxicity
DILI
- - -
Rat Oral Acute Toxicity
- -
FDAMDD
+++
Respiratory
-
Human Hepatotoxicity
+++
Ototoxicity
+++
Drug-induced Nephrotoxicity
++
Drug-induced Neurotoxicity
++
Hematotoxicity
- - -
Genotoxicity
++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C10H15NO
|
||||
| PubChem CID | |||||
| Canonical SMILES |
CC(C(C1=CC=CC=C1)O)NC
|
||||
| InChI |
1S/C10H15NO/c1-8(11-2)10(12)9-6-4-3-5-7-9/h3-8,10-12H,1-2H3/t8-,10+/m0/s1
|
||||
| InChIKey |
KWGRBVOPPLSCSI-WCBMZHEXSA-N
|
||||
| CAS Number |
CAS 90-82-4
|
||||
| ChEBI ID | |||||
| Herb ID | |||||
| SymMap ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Domperidone | Digestive system disease | Click to Show/Hide the Molecular Data of This Drug | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| Experimental
Result(s) |
The combination of domperidone and pseudoephedrine improved self reported snoring and sleepiness, and may have improved apneic episodes and sleep-related nocturnal oxygen desaturation in patients with obstructive sleep apnea provided the proportion of time spent asleep did not diminish. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Dopamine D2 receptor (D2R) | Molecule Info | [3] | |
| KEGG Pathway | Rap1 signaling pathway | Click to Show/Hide | ||
| 2 | cAMP signaling pathway | |||
| 3 | Neuroactive ligand-receptor interaction | |||
| 4 | Gap junction | |||
| 5 | Dopaminergic synapse | |||
| 6 | Parkinson's disease | |||
| 7 | Cocaine addiction | |||
| 8 | Alcoholism | |||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | Click to Show/Hide | ||
| 2 | Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | |||
| 3 | Dopamine receptor mediated signaling pathway | |||
| 4 | Nicotine pharmacodynamics pathway | |||
| Reactome | Dopamine receptors | Click to Show/Hide | ||
| 2 | G alpha (i) signalling events | |||
| WikiPathways | Hypothetical Network for Drug Addiction | Click to Show/Hide | ||
| 2 | Monoamine GPCRs | |||
| 3 | GPCRs, Class A Rhodopsin-like | |||
| 4 | Genes and (Common) Pathways Underlying Drug Addiction | |||
| 5 | GPCR ligand binding | |||
| 6 | GPCR downstream signaling | |||
| 7 | Nicotine Activity on Dopaminergic Neurons | |||

