Drug Details
| General Information of the Drug (ID: DR2599) | ||||
|---|---|---|---|---|
| Name |
Vecuronium
|
|||
| Synonyms |
Vecuronium; 86029-43-8; CHEBI:9939; UNII-5438723848; Piperidinium, 1-((2beta,3alpha,5alpha,16beta,17beta)-3,17-bis(acetyloxy)-2-(1-piperidinyl)androstan-16-yl)-1-methyl-; 50700-72-6; ORG-NC 45; 1-[(1S,2S,4S,5S,7S,10R,11S,13S,14R,15S)-5,14-bis(acetyloxy)-2,15-dimethyl-4-(piperidin-1-yl)tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-13-yl]-1-methylpiperidin-1-ium; NCGC00167467-01; Musculax; Vecuronium cation; Epitope ID:116647; GTPL4002; CHEMBL1201219; DTXSID1044146; SCHEMBL13575838; HMS2090D22; ZINC4097404; BDBM50424713; ORG NC 45 / ORG-NC 45; AKOS025290368; DB01339; MCULE-4520566918; (2beta,3alpha,5alpha,16beta,17beta)-3,17-diacetoxy-16-(1-methylpiperidinium-1-yl)-2-(piperidin-1-yl)androstane; C07553; AB00698366-05; AB00698366-07; AB00698366_08; Q421224; BRD-K20168442-004-06-3
Click to Show/Hide
|
|||
| Molecular Type |
Small molecule
|
|||
| Disease | Tonus and reflex abnormality [ICD-11: MB47] | Approved | [1] | |
| Structure |
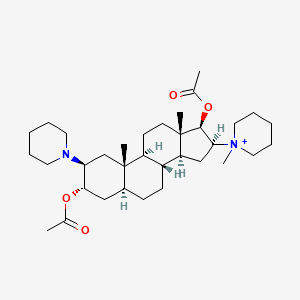
|
Click to Download Mol2D MOL |
||
| ADMET Property |
BDDCS Class
Biopharmaceutics Drug Disposition Classification System (BDDCS) Class 3: high solubility and low permeability
Clearance
The drug present in the plasma can be removed from the body at the rate of 4.5 mL/min/kg
Elimination
20% of drug is excreted from urine in the unchanged form
Half-life
The concentration or amount of drug in body reduced by one-half in 51C80 minutes
MRTD
The Maximum Recommended Therapeutic Dose (MRTD) of drug that ensured maximising efficacy and moderate side effect is 0.157 micromolar/kg/day
Unbound Fraction
The unbound fraction of drug in plasma is 0.25%
Vd
Fluid volume that would be required to contain the amount of drug present in the body at the same concentration as in the plasma 0.3 L/kg
Click to Show/Hide
|
|||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | ||||
| Formula |
C34H57N2O4+
|
|||
| PubChem CID | ||||
| Canonical SMILES |
CC(=O)OC1CC2CCC3C(C2(CC1N4CCCCC4)C)CCC5(C3CC(C5OC(=O)C)[N+]6(CCCCC6)C)C
|
|||
| InChI |
1S/C34H57N2O4/c1-23(37)39-31-20-25-12-13-26-27(34(25,4)22-29(31)35-16-8-6-9-17-35)14-15-33(3)28(26)21-30(32(33)40-24(2)38)36(5)18-10-7-11-19-36/h25-32H,6-22H2,1-5H3/q+1/t25-,26+,27-,28-,29-,30-,31-,32-,33-,34-/m0/s1
|
|||
| InChIKey |
BGSZAXLLHYERSY-XQIGCQGXSA-N
|
|||
| CAS Number |
CAS 86029-43-8
|
|||
| ChEBI ID | ||||
| TTD Drug ID | ||||
| DrugBank ID | ||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Natural Product(s) Able to Enhance the Efficacy of This Drug | ||||||
| d-tubocurarine | Chondrodendron tomentosum | Click to Show/Hide the Molecular Data of This NP | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Clinical trial | |||||
| Experimental
Result(s) |
Combination of vecuronium plus d-tubocurarine to be significantly more potent than would be expected from a simple additive effect of the individual drugs given alone. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Neuronal acetylcholine receptor alpha-2 (CHRNA2) | Molecule Info | [3] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | Click to Show/Hide | ||
| Panther Pathway | Nicotinic acetylcholine receptor signaling pathway | Click to Show/Hide | ||
| Reactome | Highly calcium permeable postsynaptic nicotinic acetylcholine receptors | Click to Show/Hide | ||
| 2 | Highly calcium permeable nicotinic acetylcholine receptors | |||
| WikiPathways | Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | Click to Show/Hide | ||

