Natural Product (NP) Details
| General Information of the NP (ID: NP5254) | |||||
|---|---|---|---|---|---|
| Name |
d-tubocurarine
|
||||
| Synonyms |
tubocurarine; d-Tubocurarine; Tubocurarin; Tubocurarine chloride; (+)-Tubocurarine; Tubocurarinum; Delacurarine; Tubarine; Isoquinoline alkaloid; 57-95-4; D-Tubocurarine chloride; UNII-W9YXS298BM; CHEBI:9774; (+)-Tubocurarine chloride; 7',12'-Dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyltubocuraranium; W9YXS298BM; 6989-98-6; Jexin; (+) Tubocurarine; (1S,16R)-9,21-dihydroxy-10,25-dimethoxy-15,15,30-trimethyl-7,23-dioxa-15,30-diazaheptacyclo[22.6.2.2^{3,6}.1^{8,12}.1^{18,22}.0^{27,31}.0^{16,34}]hexatriaconta-3,5,8,10,12(34),18(33),19,21,24(32),25,27(31),35-dodecaen-15-ium; 7',12'-dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyltubocuraran-2'-ium; NCGC00163242-01; HSDB 2152; Tubocurarine, (+)-; Spectrum_001966; SpecPlus_000475; Spectrum2_001335; Spectrum3_001095; Spectrum4_001922; Spectrum5_000685; Epitope ID:174836; dimethoxy(trimethyl)[?]diol; BSPBio_002770; KBioGR_002264; KBioSS_002526; MLS003882581; DivK1c_006571; SCHEMBL121375; SPBio_001489; CHEMBL339427; GTPL2294; DTXSID0048393; KBio1_001515; KBio2_002518; KBio2_005086; KBio2_007654; KBio3_001990; HMS2089C06; ZINC3978083; BDBM50366799; PDSP1_001485; PDSP2_001469; Tubocuraranium, 7',12'-dihydroxy-6,6'-dimethoxy-2,2',2'-trimethyl-; DB01199; SDCCGMLS-0066631.P001; NCGC00163242-02; NCGC00178480-01; 13H-4,6:21,24-Dietheno-8,12-metheno-1H-pyrido(3',2':14,15)(1,11)dioxacycloeicosino(2,3,4-ij)isoquinolinium, 2,3,13a,14,15,16,25,25a-octahydro-9,19-dihydroxy-18,29-dimethoxy-1,14,14-trimethyl-, (13aR-(13aR*,25aS*))-; SMR002533646; SBI-0052455.P002; C07547; AB00053831-03; AB00053831_04; Q421268; SR-05000001878-4; BRD-K99621550-003-03-4; 13H-4,6:21,24-Dietheno-8,12-metheno-1H-pyrido(3',2':14,15)(1,11)dioxacycloeicosino(2,3,4-ij)isoquinolinium, 2,3,13a,14,15,16,25,25a-octahydro-9,19-dihydroxy-18,29-dimethoxy-1,14,14-trimethyl-, (13aR,25aS)-
Click to Show/Hide
|
||||
| Species Origin | Chondrodendron tomentosum ... | Click to Show/Hide | |||
| Chondrodendron tomentosum | |||||
| Disease | Corneal disease [ICD-11: 9A78] | Approved | [1] | ||
| Structure |
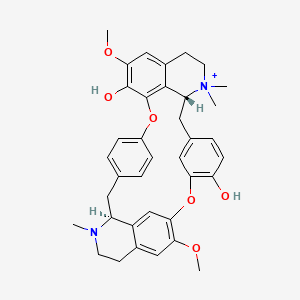
|
Click to Download Mol2D MOL |
|||
| ADMET Property |
Absporption
Caco-2 Permeability
-5.594
MDCK Permeability
-5.015
PAMPA
- - -
HIA
- -
Distribution
VDss
-0.377
PPB
47.8%
BBB
- - -
Metabolism
CYP1A2 inhibitor
- - -
CYP1A2 substrate
+++
CYP2C19 inhibitor
- - -
CYP2C19 substrate
+++
CYP2C9 inhibitor
- - -
CYP2C9 substrate
+
CYP2D6 inhibitor
- - -
CYP2D6 substrate
+++
CYP3A4 inhibitor
- - -
CYP3A4 substrate
+
CYP2B6 inhibitor
++
CYP2B6 substrate
++
CYP2C8 inhibitor
- - -
HLM Stability
- - -
Excretion
CLplasma
3.91
T1/2
2.877
Toxicity
DILI
- - -
Rat Oral Acute Toxicity
+++
FDAMDD
+++
Respiratory
+++
Human Hepatotoxicity
- -
Ototoxicity
- -
Drug-induced Nephrotoxicity
- -
Drug-induced Neurotoxicity
++
Hematotoxicity
- - -
Genotoxicity
++
Tips: 1. For the classification endpoints, the prediction probability values are transformed into six symbols: 0-0.1 (- - -), 0.1-0.3 (- -), 0.3-0.5 (-), 0.5-0.7 (+), 0.7-0.9 (++), and 0.9-1.0 (+++).
2. Additionally, the corresponding relationships of the three labels are as follows: excellent; medium; poor.
Click to Show/Hide
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Natural Product | |||||
| Formula |
C37H41N2O6+
|
||||
| PubChem CID | |||||
| Canonical SMILES |
CN1CCC2=CC(=C3C=C2C1CC4=CC=C(C=C4)OC5=C6C(CC7=CC(=C(C=C7)O)O3)[N+](CCC6=CC(=C5O)OC)(C)C)OC
|
||||
| InChI |
1S/C37H40N2O6/c1-38-14-12-24-19-32(42-4)33-21-27(24)28(38)16-22-6-9-26(10-7-22)44-37-35-25(20-34(43-5)36(37)41)13-15-39(2,3)29(35)17-23-8-11-30(40)31(18-23)45-33/h6-11,18-21,28-29H,12-17H2,1-5H3,(H-,40,41)/p+1/t28-,29+/m0/s1
|
||||
| InChIKey |
JFJZZMVDLULRGK-URLMMPGGSA-O
|
||||
| CAS Number |
CAS 57-95-4
|
||||
| ChEBI ID | |||||
| Herb ID | |||||
| TTD Drug ID | |||||
| Combinatorial Therapeutic Effect(s) Validated Clinically or Experimentally | ||||||
|---|---|---|---|---|---|---|
| α. A List of Drug(s) Whose Efficacy can be Enhanced by This NP | ||||||
| Vecuronium | Tonus and reflex abnormality | Click to Show/Hide the Molecular Data of This Drug | ||||
| Achieving Therapeutic Synergy | Click to Show/Hide | |||||
| Representative Experiment Reporting the Effect of This Combination | [2] | |||||
| Detail(s) |
Combination Info
 click to show the detail info of this combination
click to show the detail info of this combination
|
|||||
| In-vivo Model | Clinical trial | |||||
| Experimental
Result(s) |
Combination of vecuronium plus d-tubocurarine to be significantly more potent than would be expected from a simple additive effect of the individual drugs given alone. | |||||
| Target and Pathway | ||||
|---|---|---|---|---|
| Target(s) | Neuronal acetylcholine receptor alpha-2 (CHRNA2) | Molecule Info | [3] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | Click to Show/Hide | ||
| Panther Pathway | Nicotinic acetylcholine receptor signaling pathway | Click to Show/Hide | ||
| Reactome | Highly calcium permeable postsynaptic nicotinic acetylcholine receptors | Click to Show/Hide | ||
| 2 | Highly calcium permeable nicotinic acetylcholine receptors | |||
| WikiPathways | Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | Click to Show/Hide | ||

